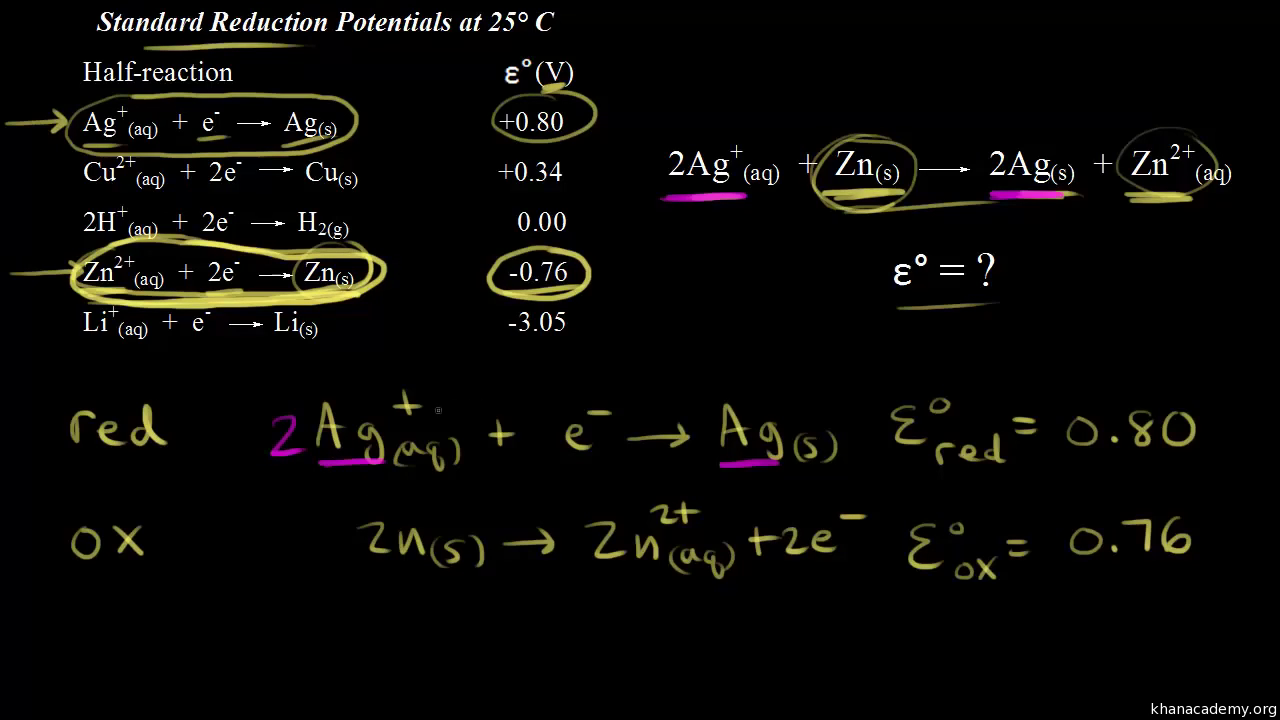
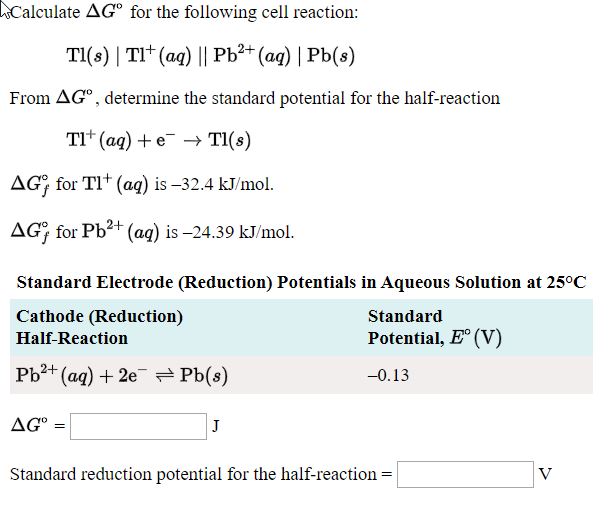
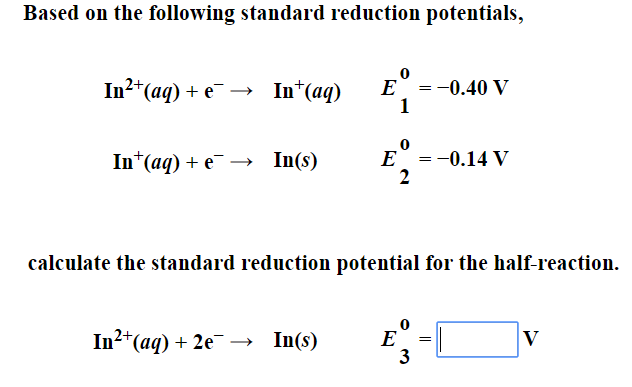
The standard reduction potential for `Cu^(2+)|Cu` is `+0.34V`. Calculate the reduction potential... - YouTube
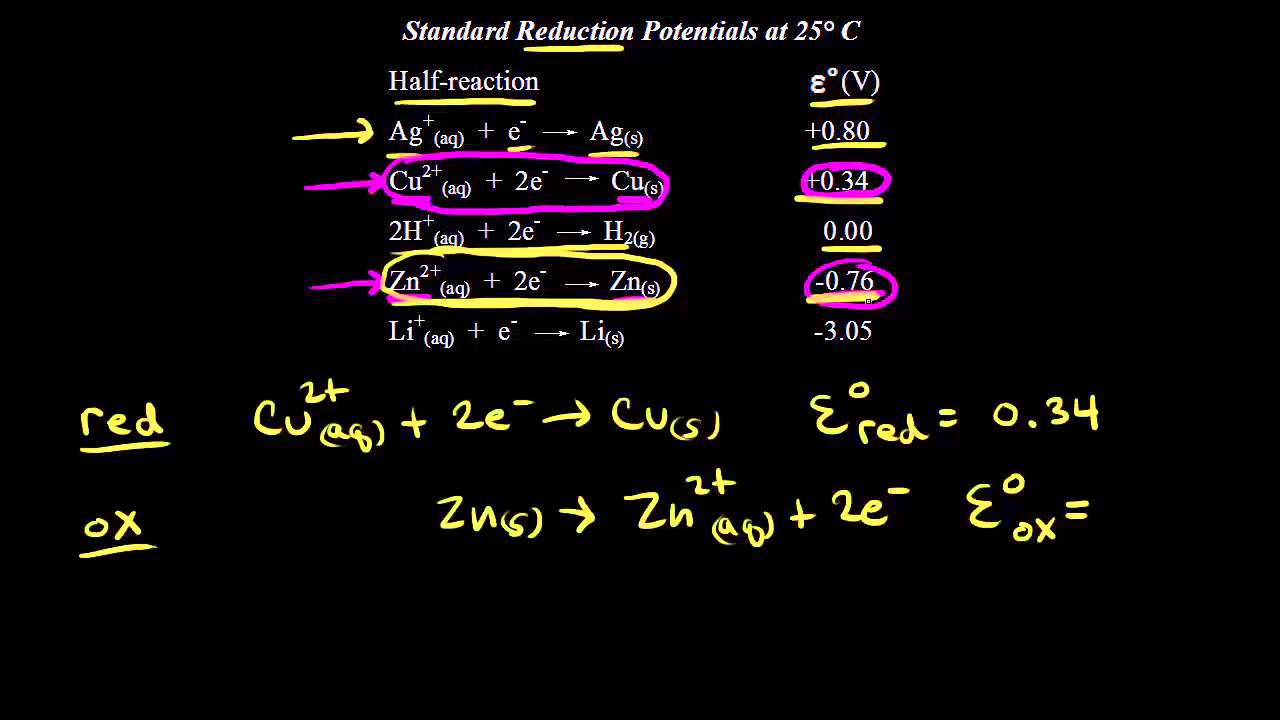
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy - YouTube

Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N-Dimethylformamide | Inorganic Chemistry

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa

The standard reduction potential for the half cell: NO3^-(aq.) + 2H^+(aq.) + e^ - → NO2(g) + H2O is 0.78 V. Calculate the reduction potential in 8M H^+ .

Calculation of Standard Reduction Potentials of Amino Acid Radicals and the Effects of Water and Incorporation into Peptides. | Semantic Scholar
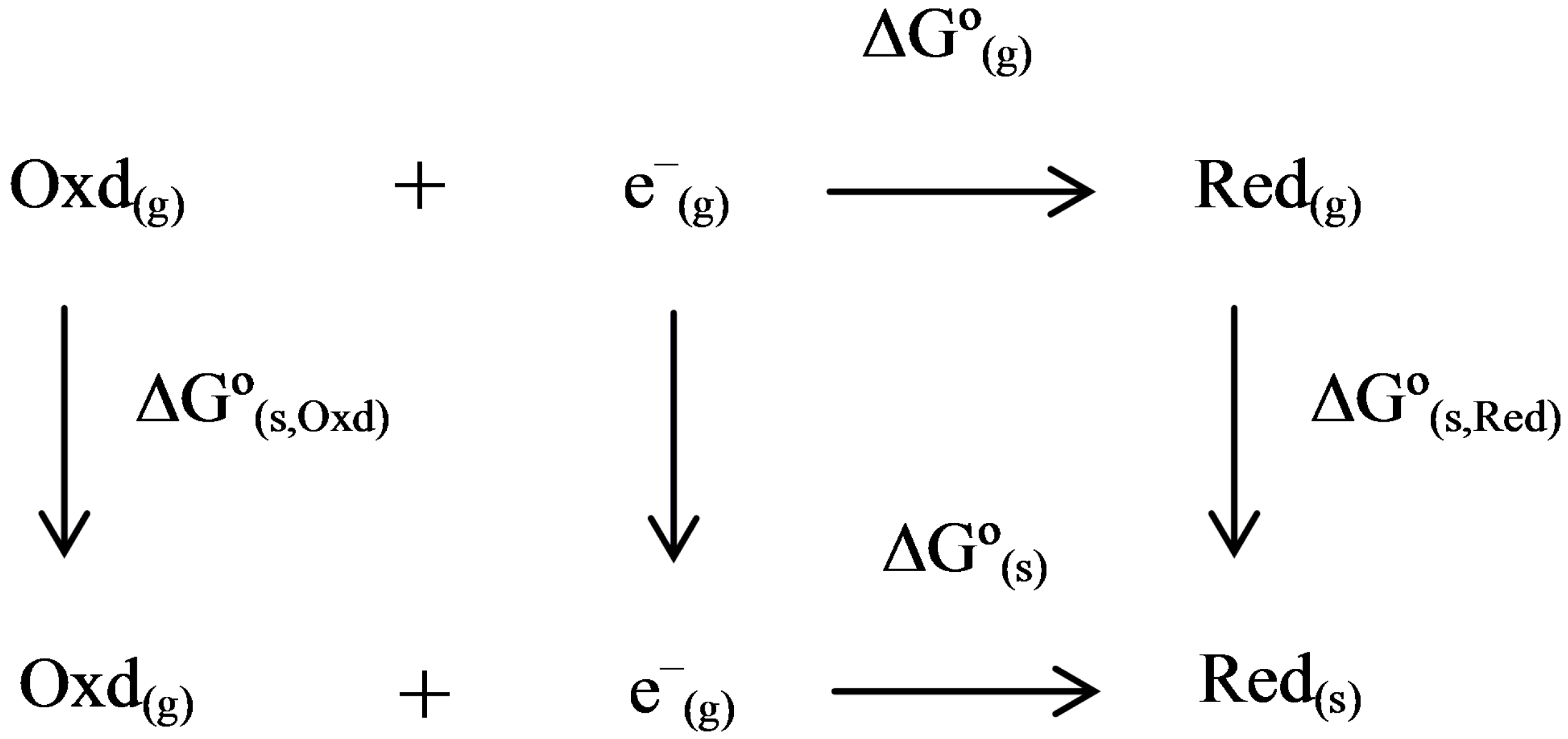
Minerals | Free Full-Text | Computational Redox Potential Predictions: Applications to Inorganic and Organic Aqueous Complexes, and Complexes Adsorbed to Mineral Surfaces

Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N-Dimethylformamide | Inorganic Chemistry
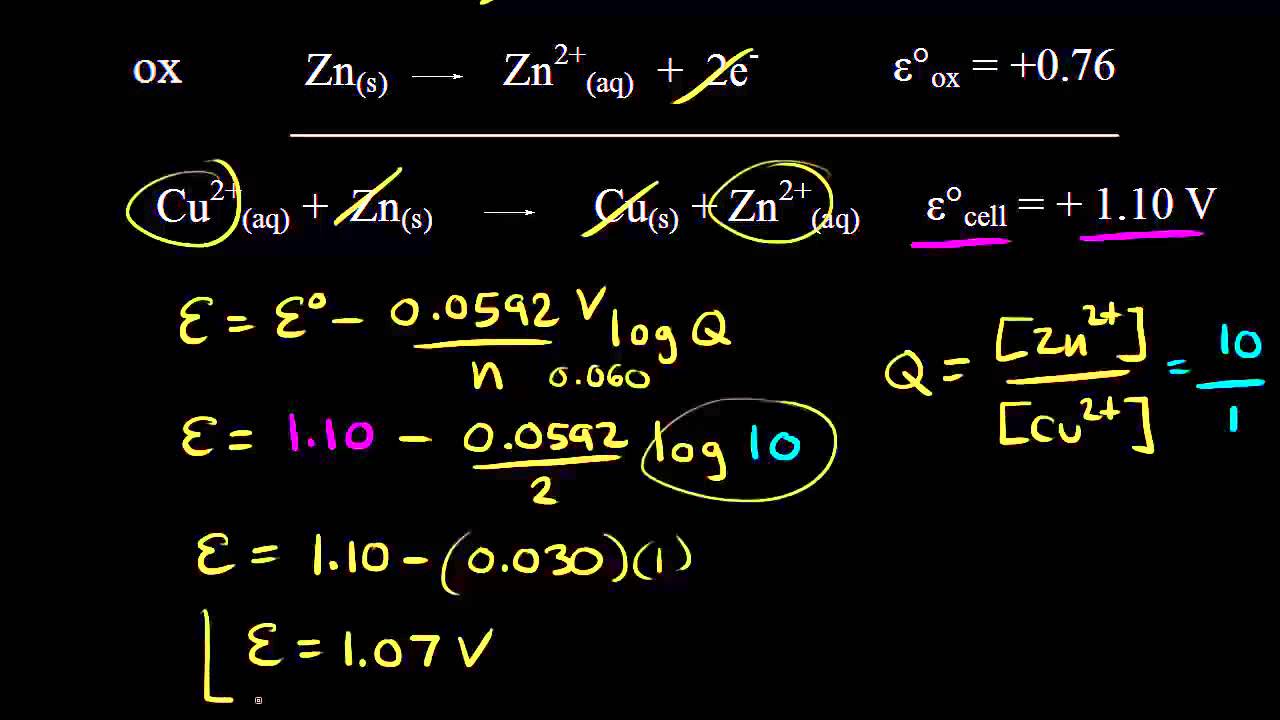
Using the Nernst equation | Redox reactions and electrochemistry | Chemistry | Khan Academy - YouTube