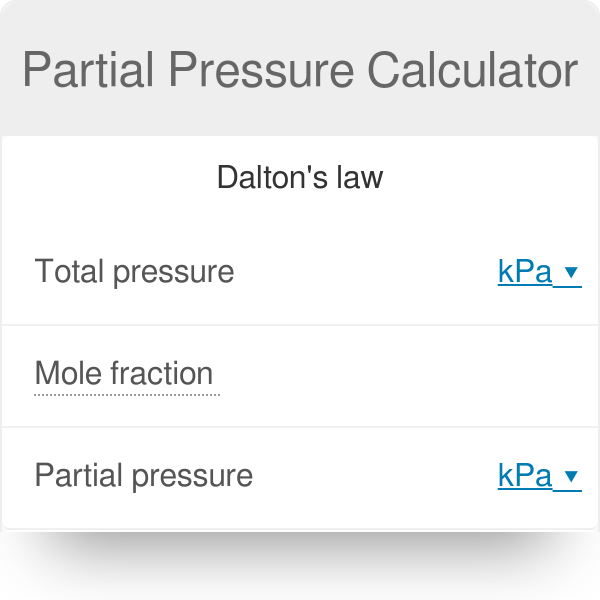
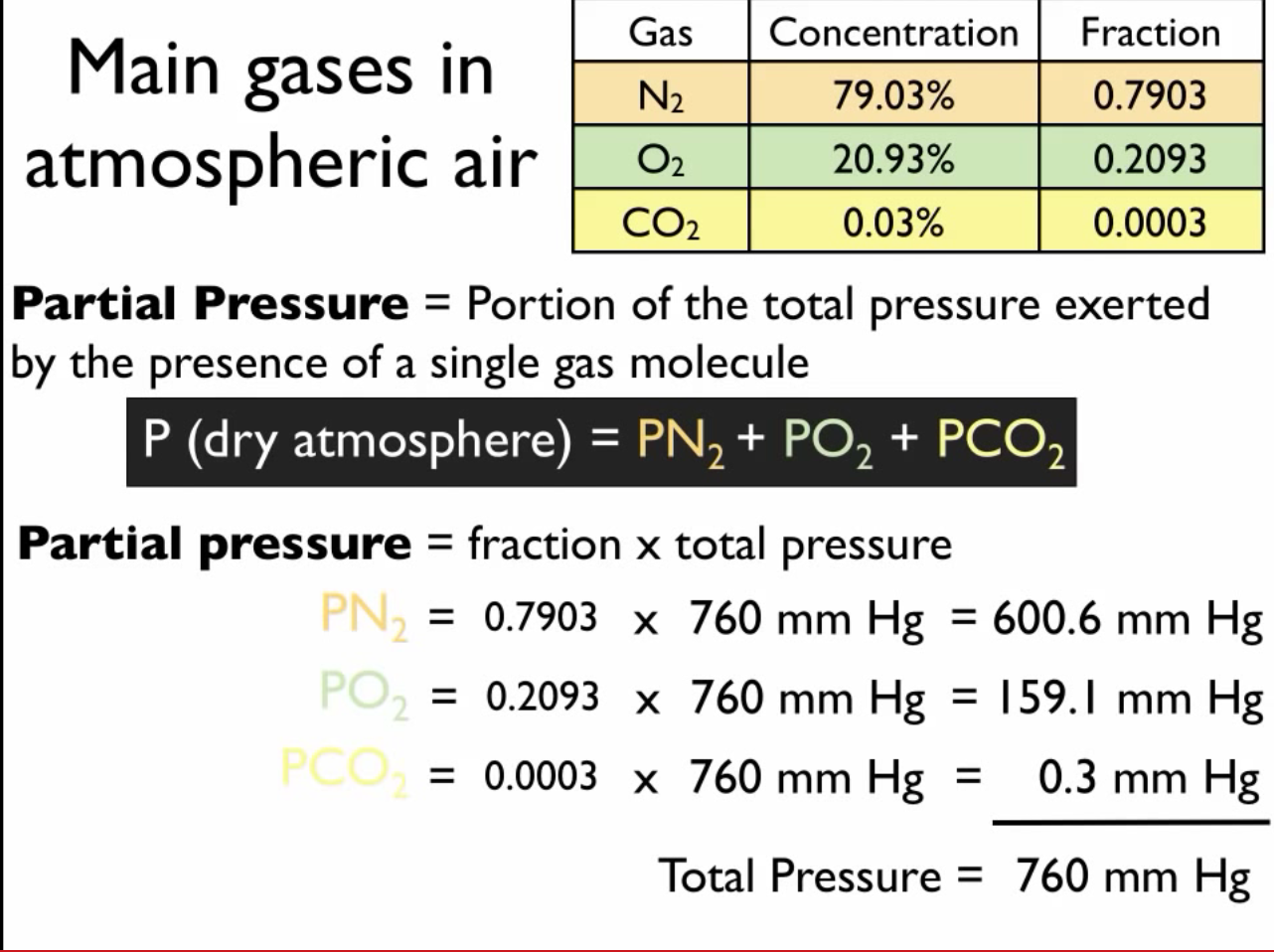
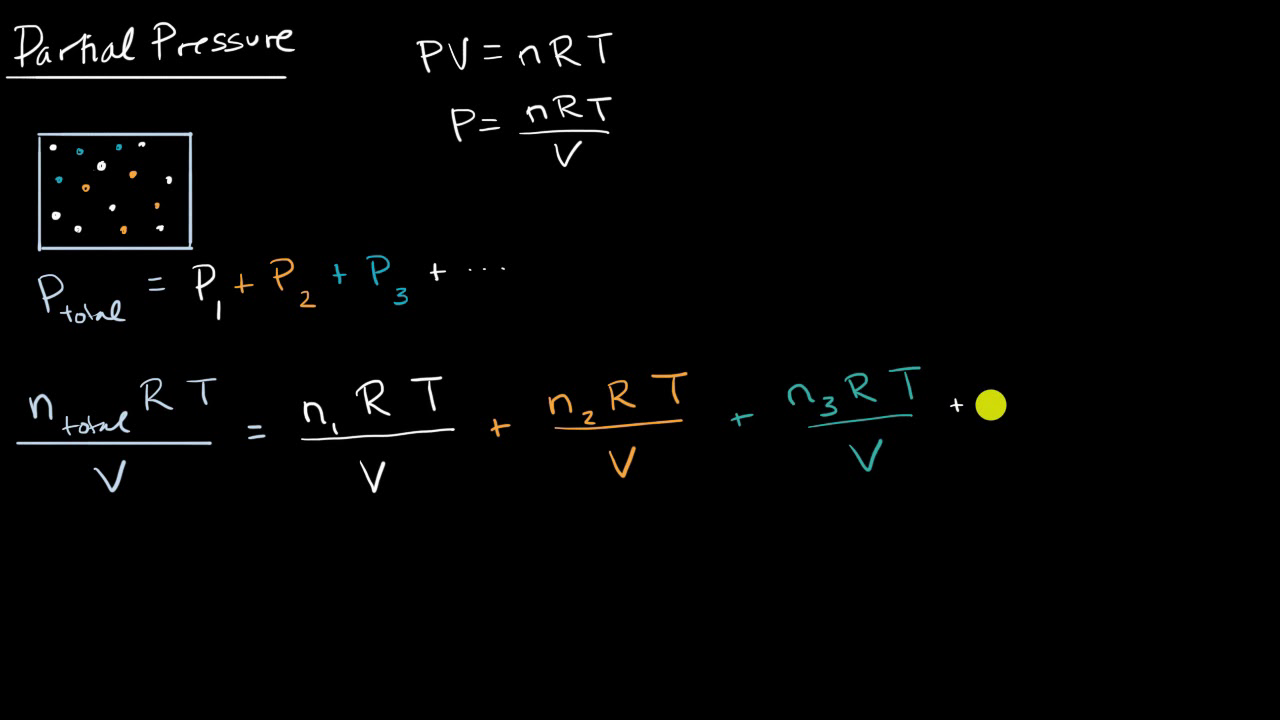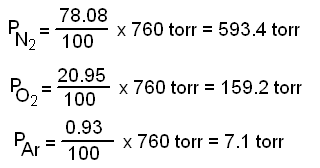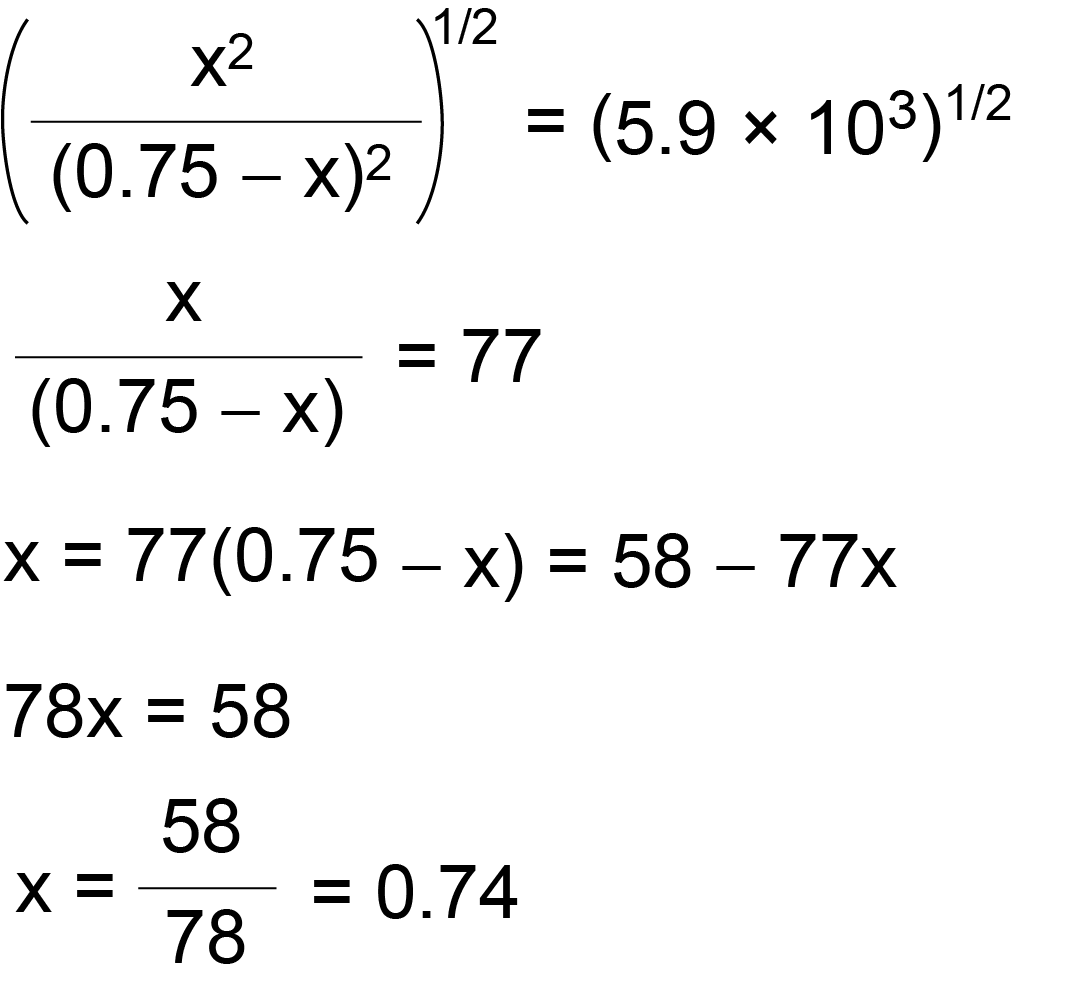Dalton's Law of Partial Pressure: Formula | How to Find Partial Pressure - Video & Lesson Transcript | Study.com

Dalton's Law of Partial Pressures/Effusion 1.Calculate and use the pressure in torr. 2.Determine the partial pressure of the light molecules. 3.Determine. - ppt download

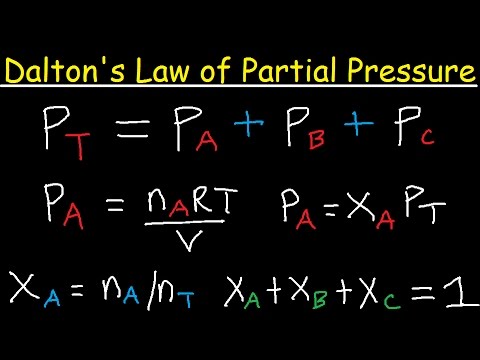
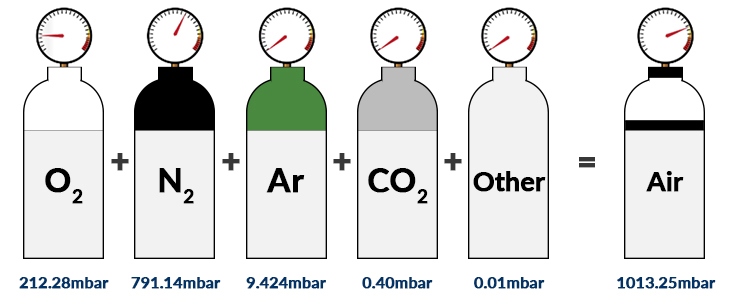
Partial Pressure- Formula, Dalton's Law, Mixture of Ideal Gas, Examples, Videos with FAQs of Partial Pressure.

Question Video: Calculating the Partial Pressure of Hydrogen Gas Given the Amount of Each Gas in the Mixture and the Total Pressure | Nagwa

Question Video: Calculating 𝐾_𝑝 at Equilibrium for a Mixture of Nitrogen, Hydrogen, and Ammonia | Nagwa
![chemistry: Dalton's Law of Partial Pressure equation] I'm trying to understand this example problem, I don't understand the question, and can't follow along with the steps : r/HomeworkHelp chemistry: Dalton's Law of Partial Pressure equation] I'm trying to understand this example problem, I don't understand the question, and can't follow along with the steps : r/HomeworkHelp](https://i.redd.it/j8g9ytpwlzb51.png)