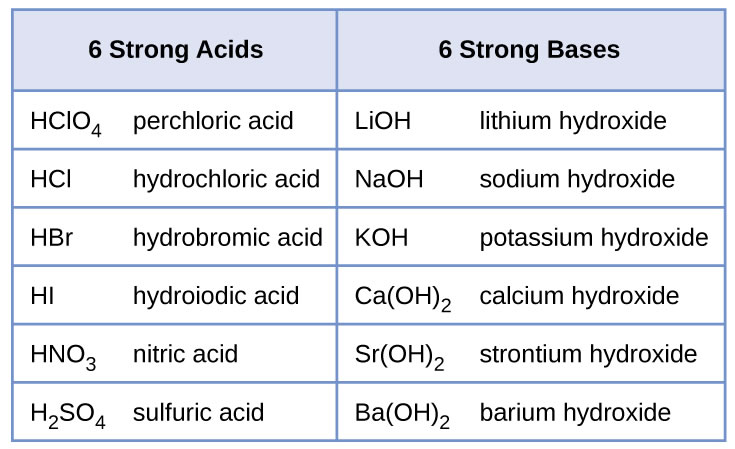
Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added to a large volume of water, one mole of what ion enters the solution? | Socratic
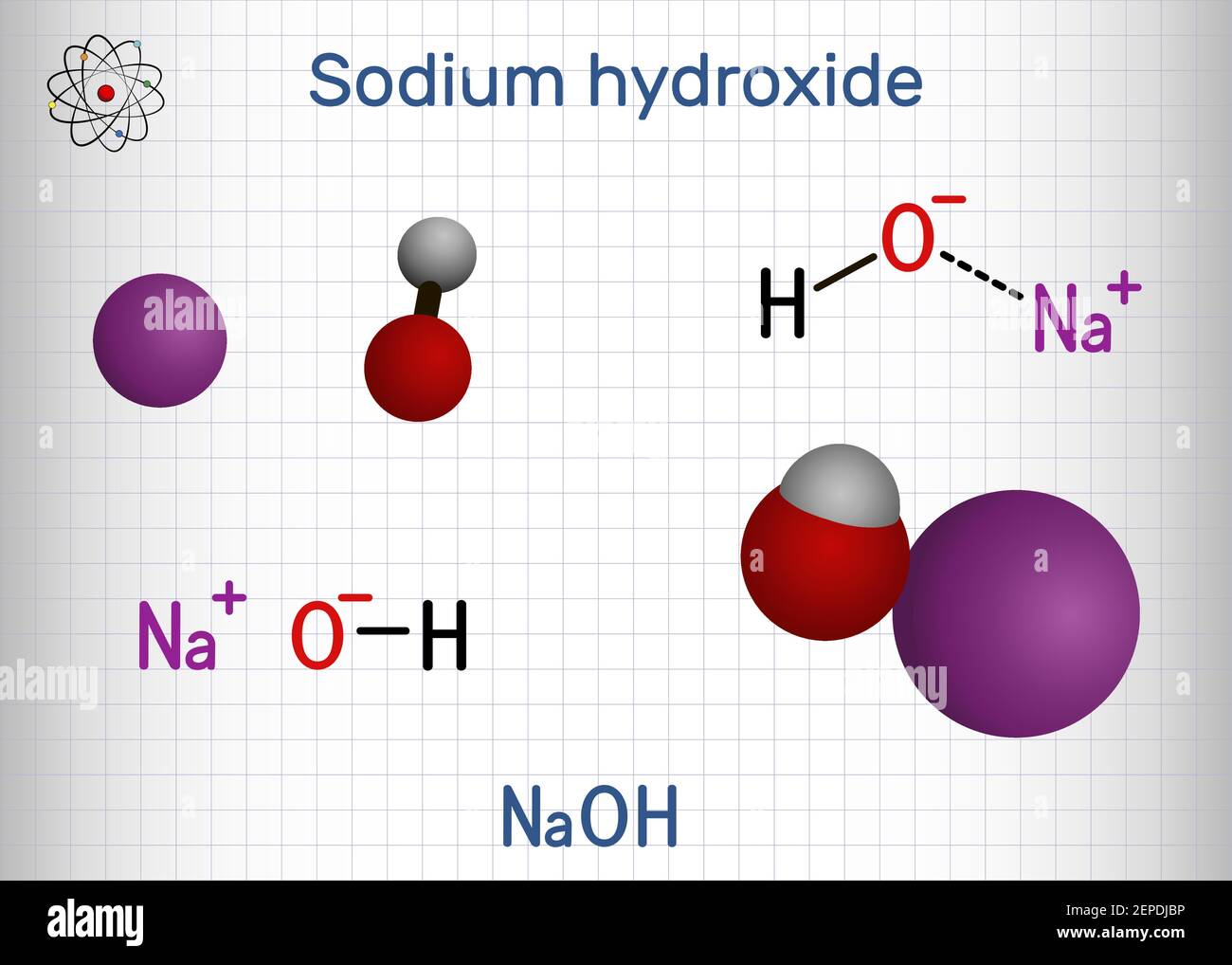
Sodium hydroxide, caustic soda, lye molecule. NaOH is highly caustic base and alkali, ionic compound. Structural chemical formula and molecule model Stock Vector Image & Art - Alamy
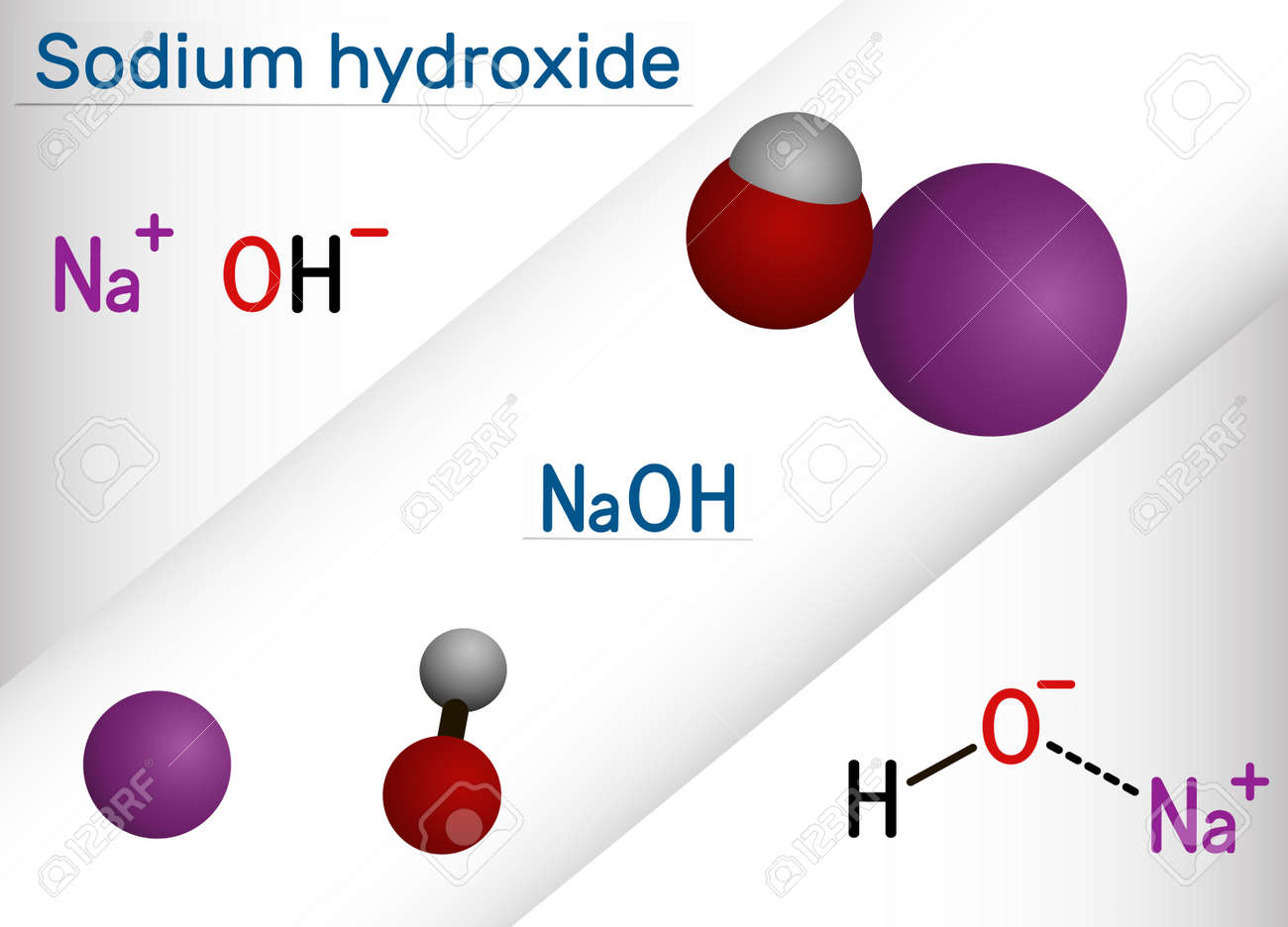
Sodium Hydroxide, Caustic Soda, Lye Molecule. NaOH Is Highly Caustic Base And Alkali, Ionic Compound. Structural Chemical Formula And Molecule Model. Vector Illustration Royalty Free SVG, Cliparts, Vectors, And Stock Illustration. Image

RICCA CHEMICAL COMPANY - Sodium Hydroxide is a strong base in terms of chemical ionization and solutions of it can be assayed using a strong acid, such as Hydrochloric Acid or Sulfuric
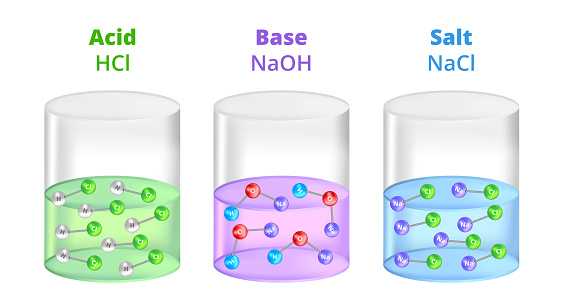
Set Of Three Chemical Containers With Acid Base And Salt With Different Ph Hcl Hydrochloric Acid Naoh Sodium Hydroxide And Nacl Sodium Chloride Stock Illustration - Download Image Now - iStock