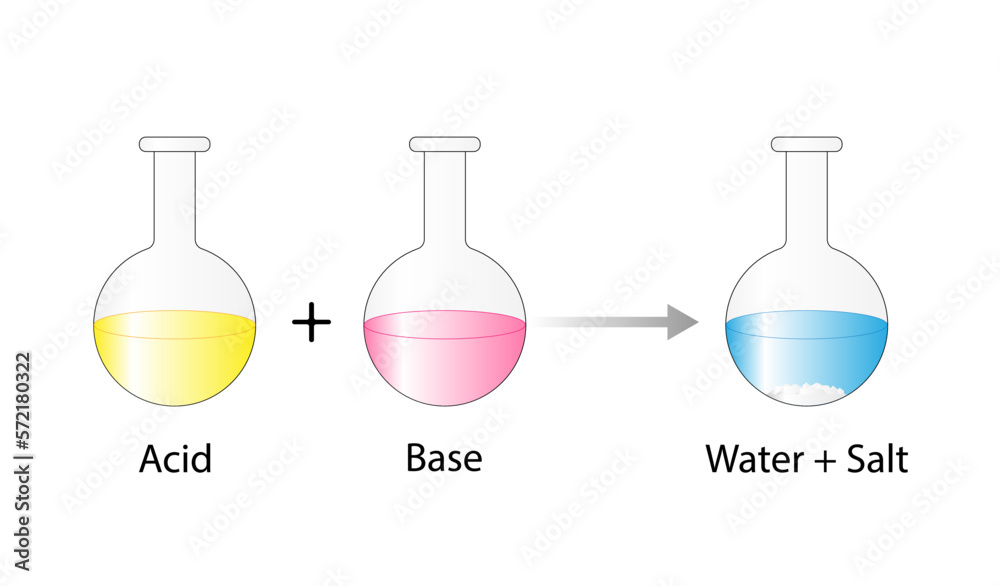
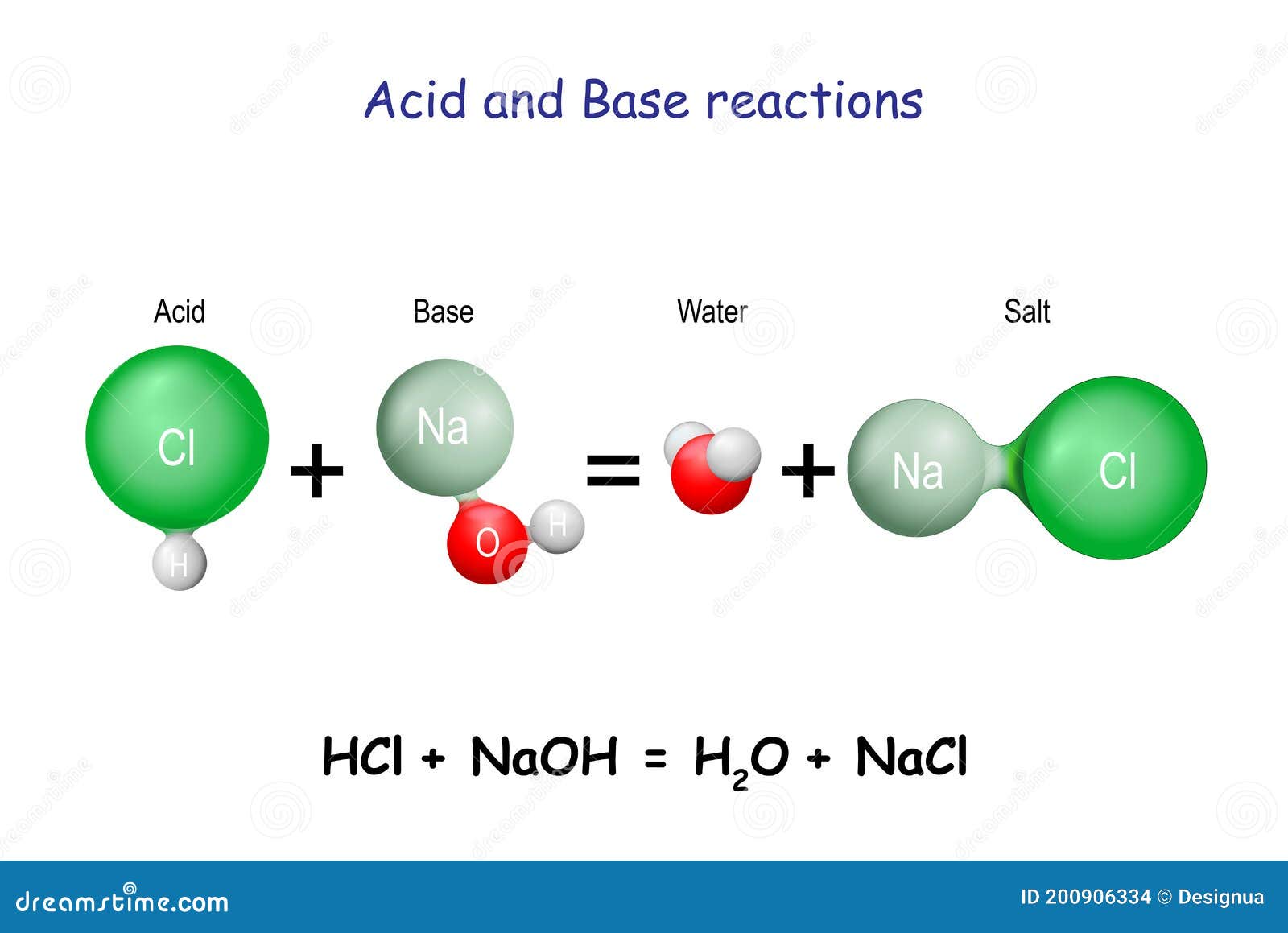
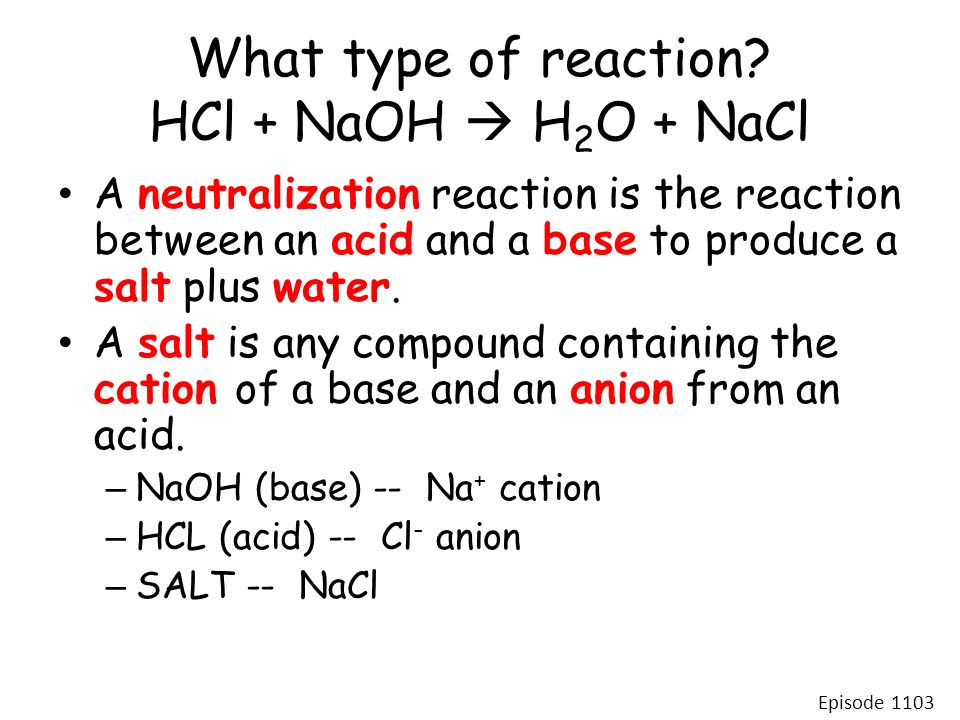
Acid–base reaction. chemical reaction neutralization. HCl hydrochloric acid, NaOH sodium hydroxide, and NaCl, sodium chloride. Vector illustration. Stock Vector | Adobe Stock

PDF) The effect of NaCl 0.9% and NaCl 0.45% on sodium, chloride, and acid– base balance in a PICU population | Almeida Loureiro - Academia.edu
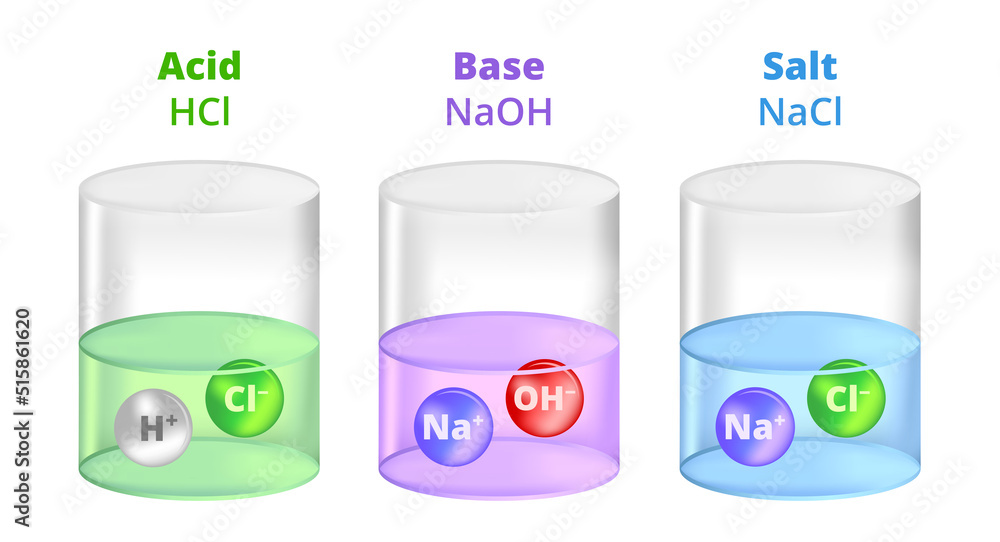
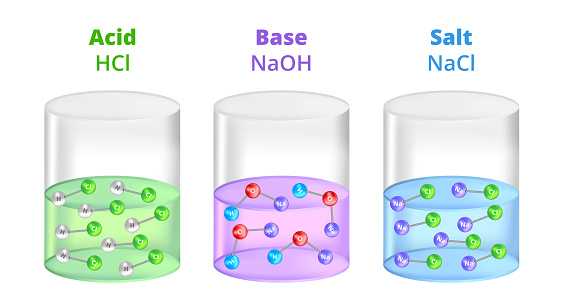
Vector illustration of electrolytic dissociation. Molecules break up into ions. Chemical containers with acid, base, and salt. HCl hydrochloric acid, NaOH sodium hydroxide, and NaCl, sodium chloride. Stock Vector | Adobe Stock

Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF
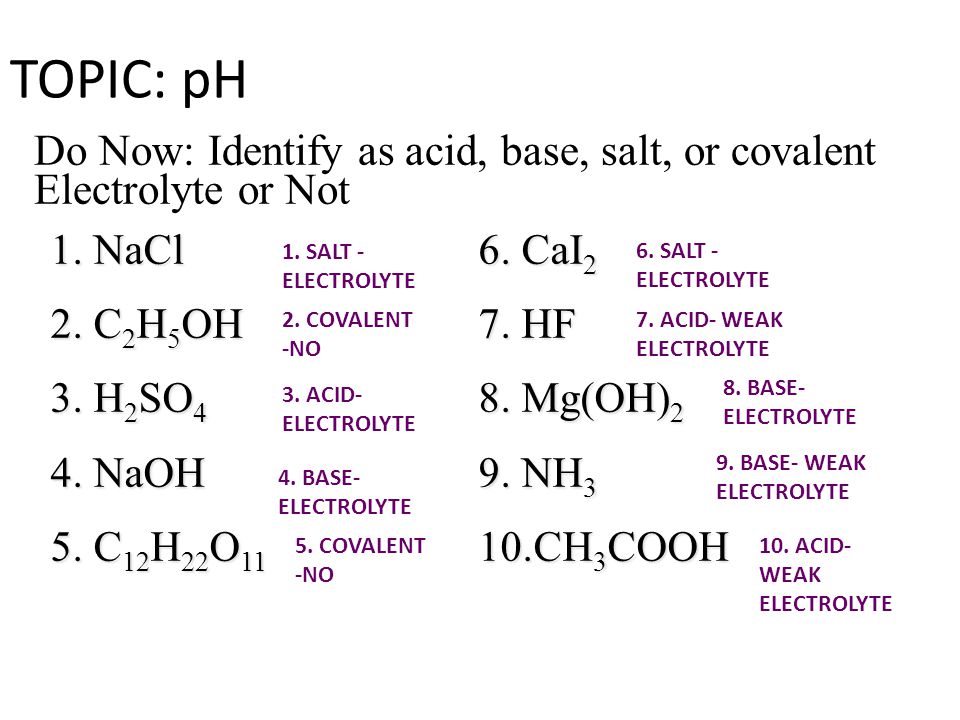
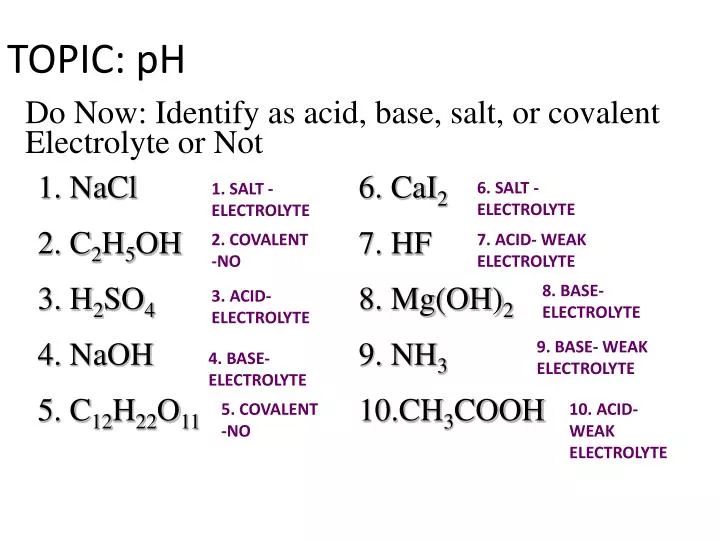
TOPIC: pH Do Now: Identify as acid, base, salt, or covalent Electrolyte or Not 1. NaCl 6. CaI2 2. C2H5OH 7. HF 3. H2SO4 8. Mg(OH)2 4. NaOH 9. NH3 5. C12H22O ppt download




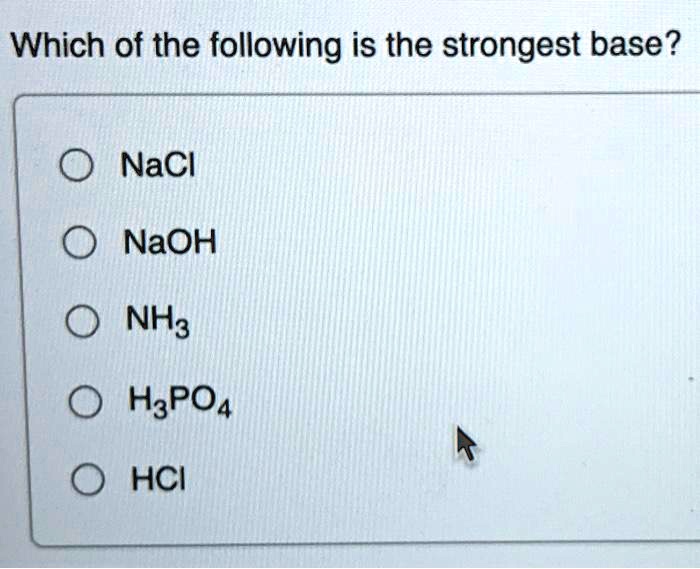







![Solved Given the pH = 7.000, [buffer] = 0.0200 M, [NaCl] = | Chegg.com Solved Given the pH = 7.000, [buffer] = 0.0200 M, [NaCl] = | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F1bb%2F1bb36f87-9687-4cb2-992a-746f0b49edbd%2FphppTOqMr.png)