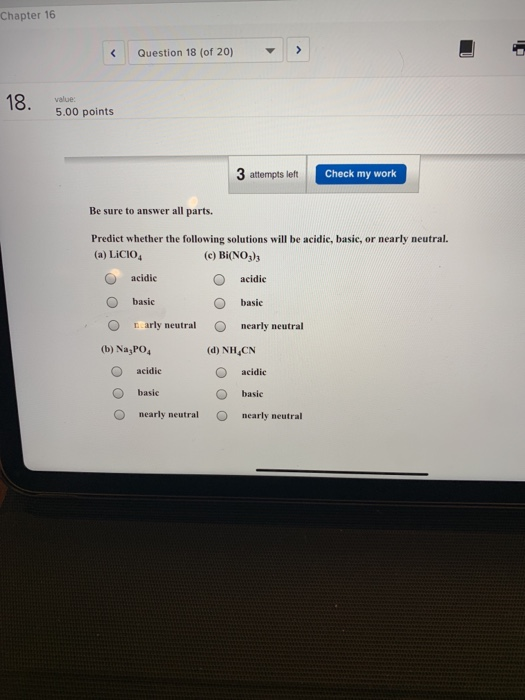
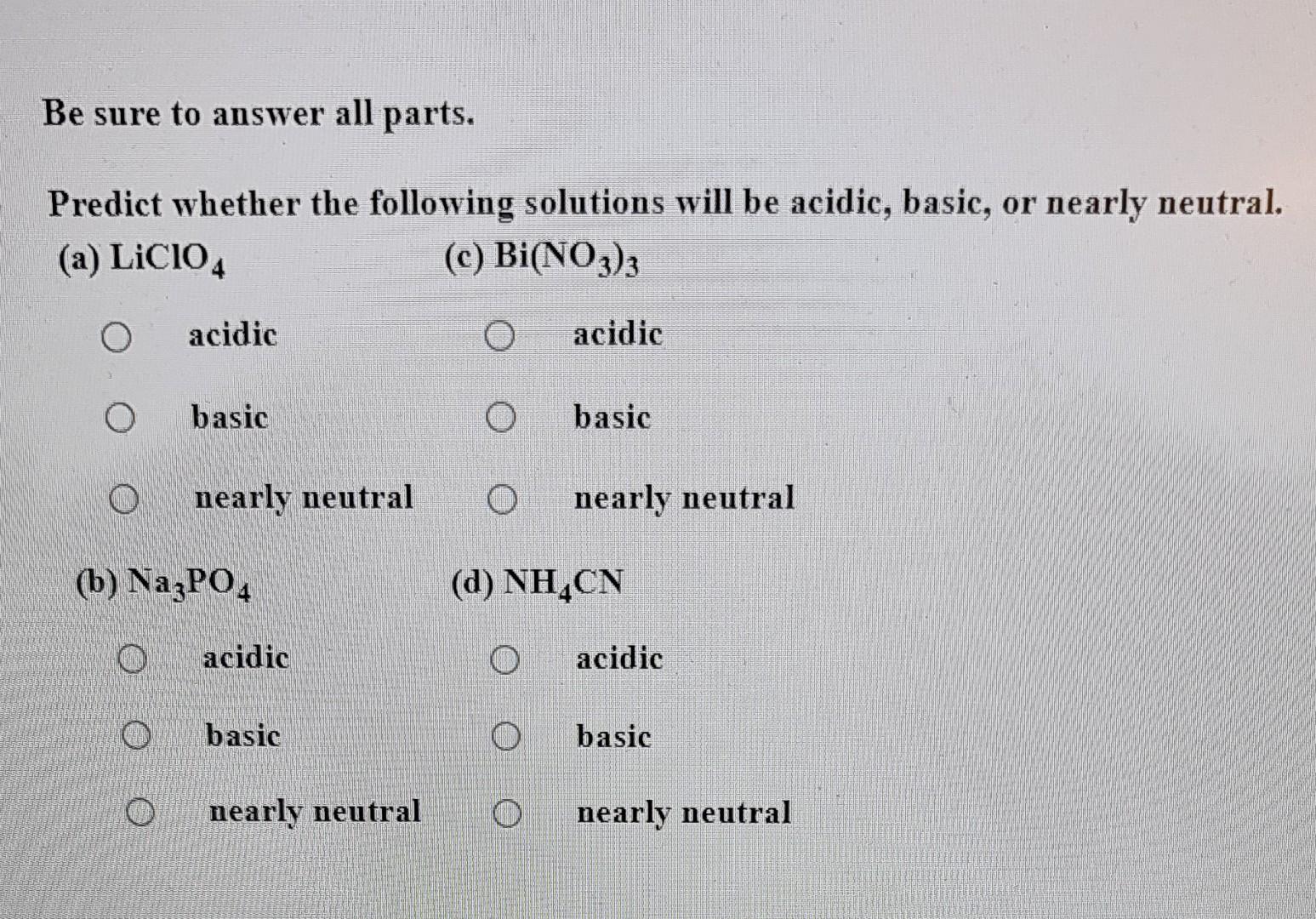
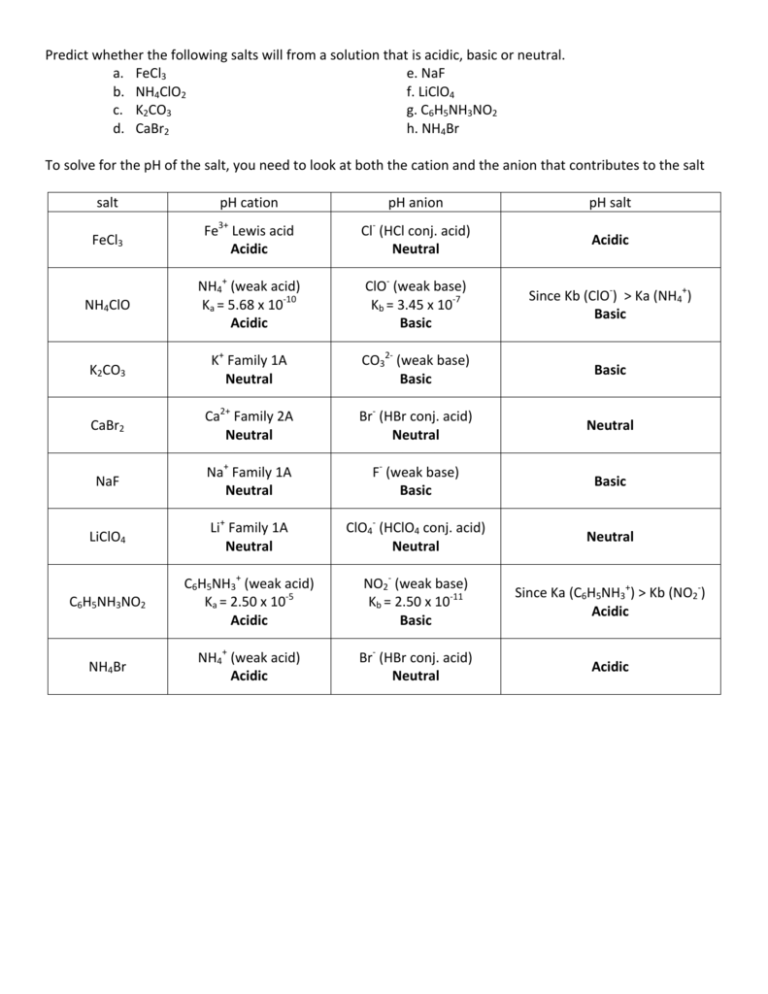
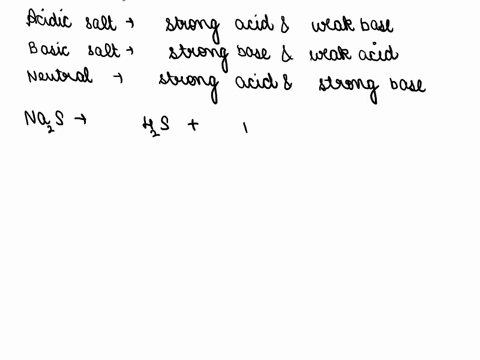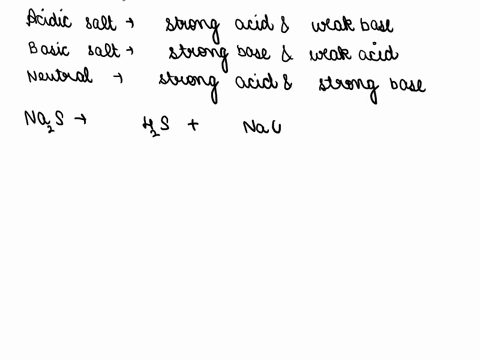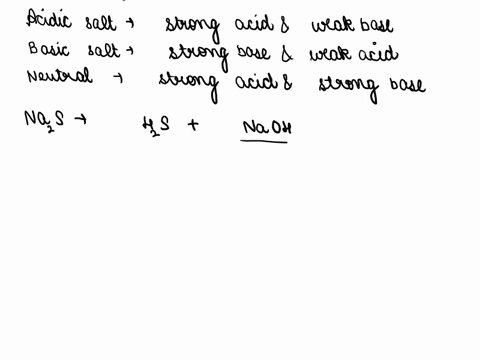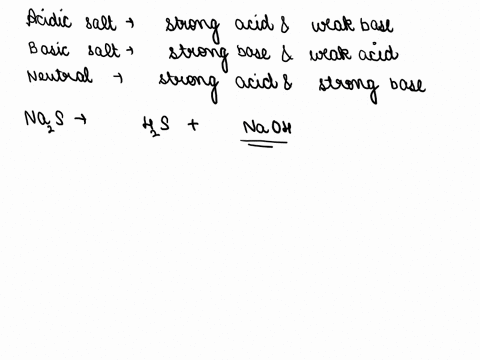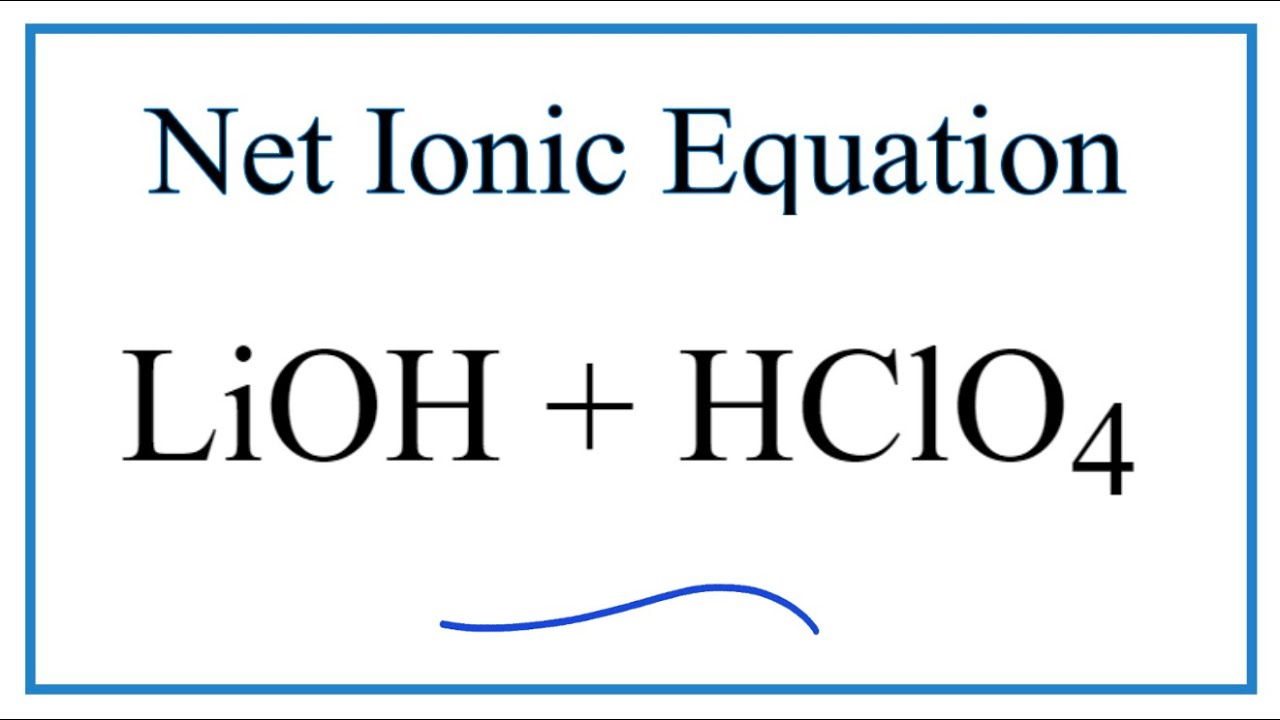Spring 2009 CH302 Worksheet 5 Answer Key—How to Systematically Work Harder and Harder Acid Base Calculations Exactly the Same
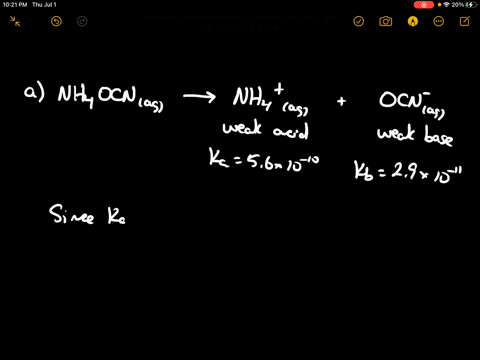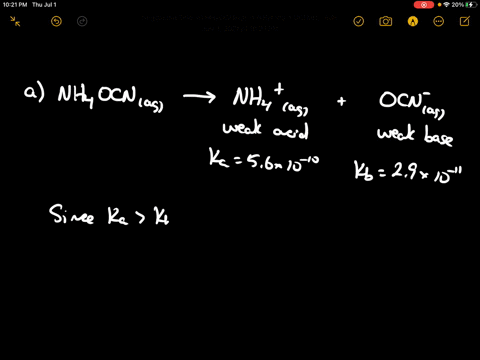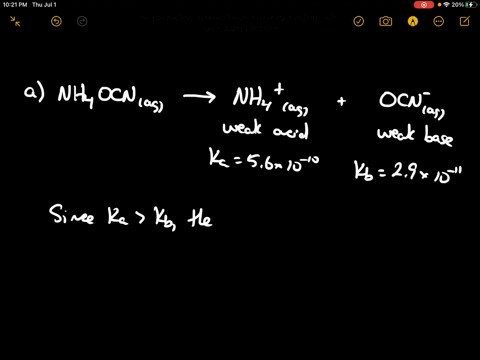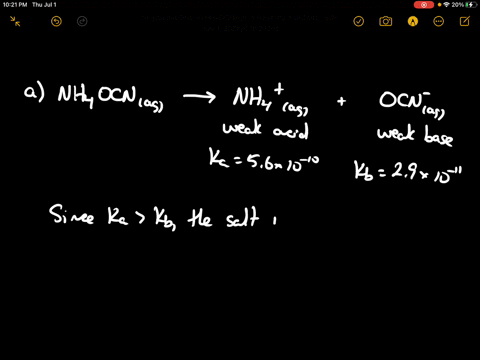
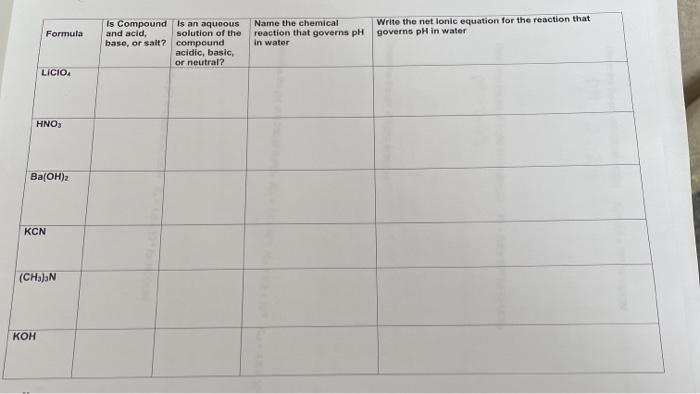
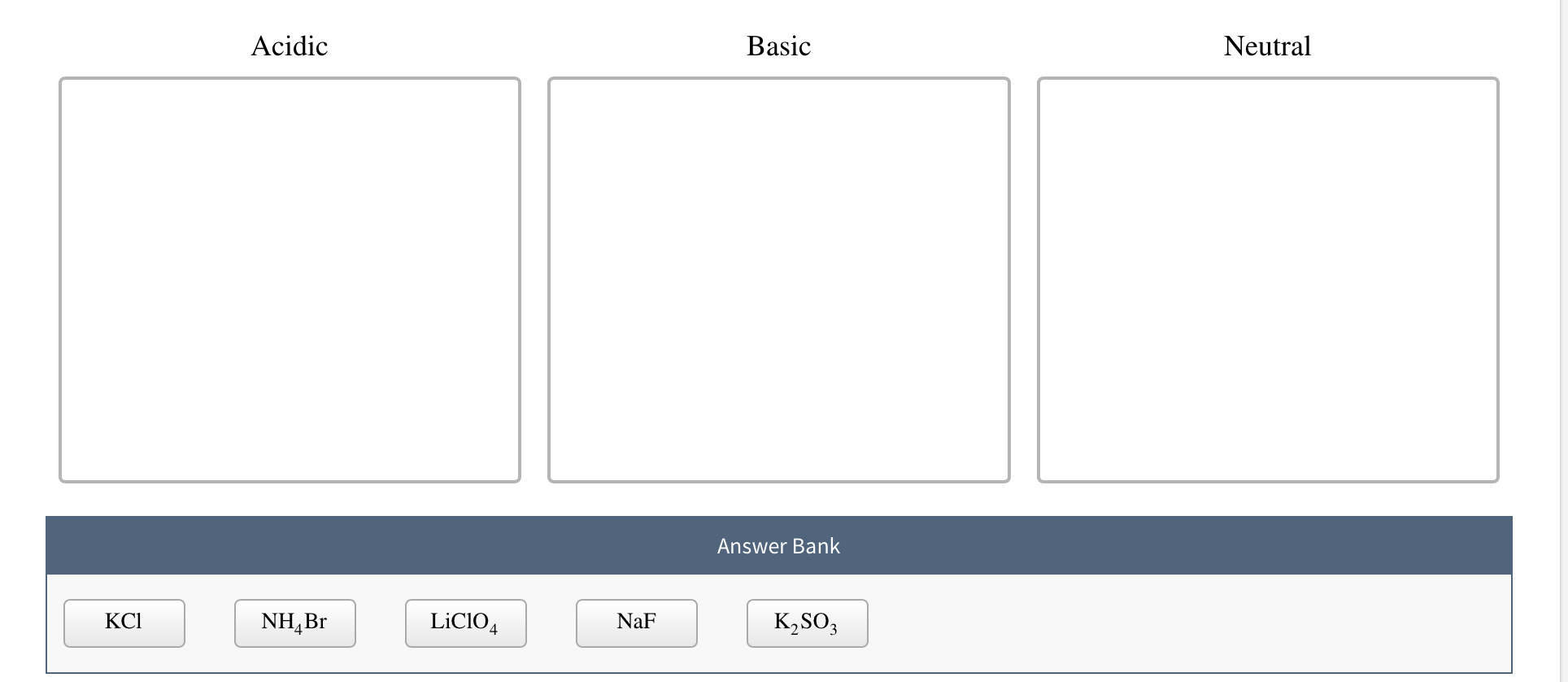
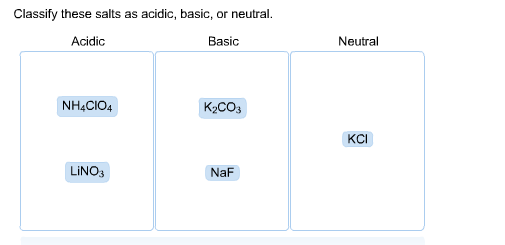
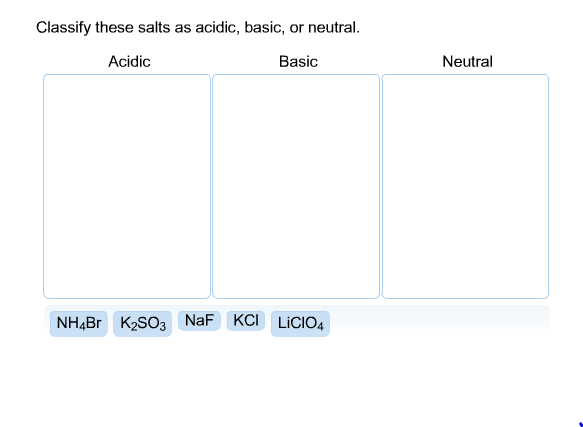
SOLVED: CLASSIFY THESE SALTS AS ACIDIC, BASIC, OR NEUTRAL. CH3CH2NH3Cl CH3CH2CO2Na HCO2K LiCLO4 CH3NH3NO3

Solvate Structures and Computational/Spectroscopic Characterization of LiClO4 Electrolytes | The Journal of Physical Chemistry C

Solvate Structures and Computational/Spectroscopic Characterization of LiClO4 Electrolytes | The Journal of Physical Chemistry C
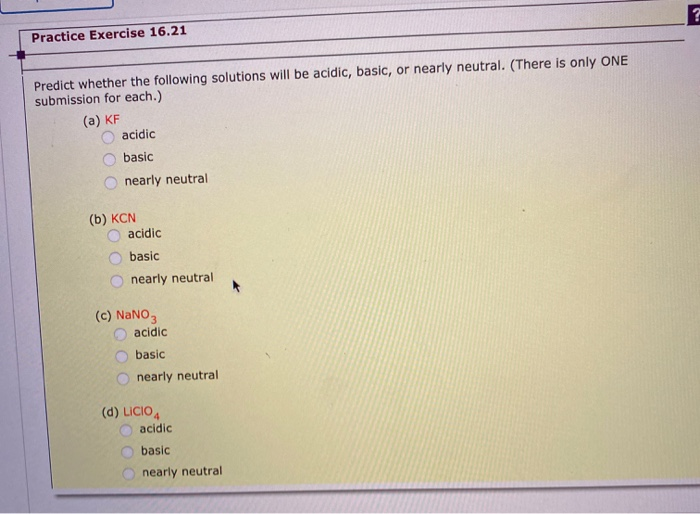
![SOLVED: 1. Identify each salt as acidic, basic, Or neutral: LiClO4 CN;CHNH;NOz Ca(QCHz 2 . Rank the following solutions by increasing [H;O-] KNO3, HQB:; NHCL; LiCH;COO, NaOH, Ca(OH)2 3. Calculate the pH SOLVED: 1. Identify each salt as acidic, basic, Or neutral: LiClO4 CN;CHNH;NOz Ca(QCHz 2 . Rank the following solutions by increasing [H;O-] KNO3, HQB:; NHCL; LiCH;COO, NaOH, Ca(OH)2 3. Calculate the pH](https://cdn.numerade.com/ask_images/756701414ae8442ab9499760d97abb5b.jpg)
SOLVED: 1. Identify each salt as acidic, basic, Or neutral: LiClO4 CN;CHNH;NOz Ca(QCHz 2 . Rank the following solutions by increasing [H;O-] KNO3, HQB:; NHCL; LiCH;COO, NaOH, Ca(OH)2 3. Calculate the pH