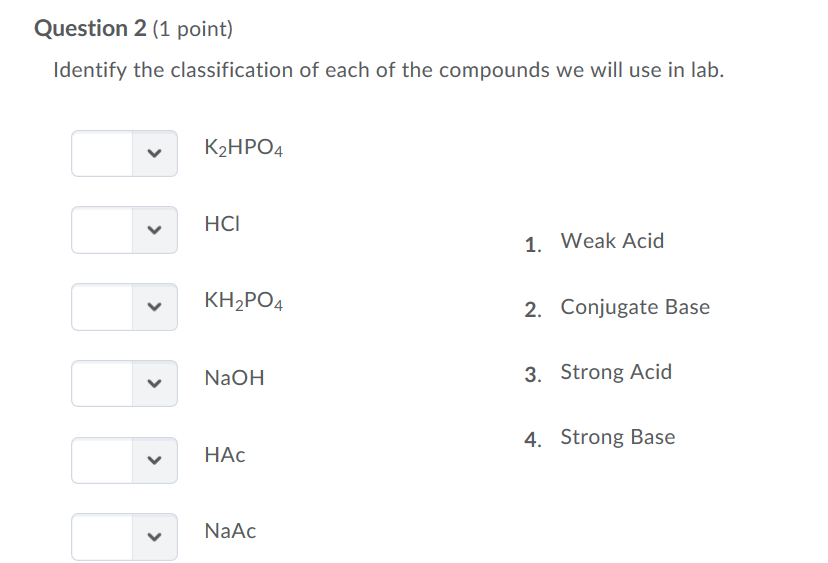
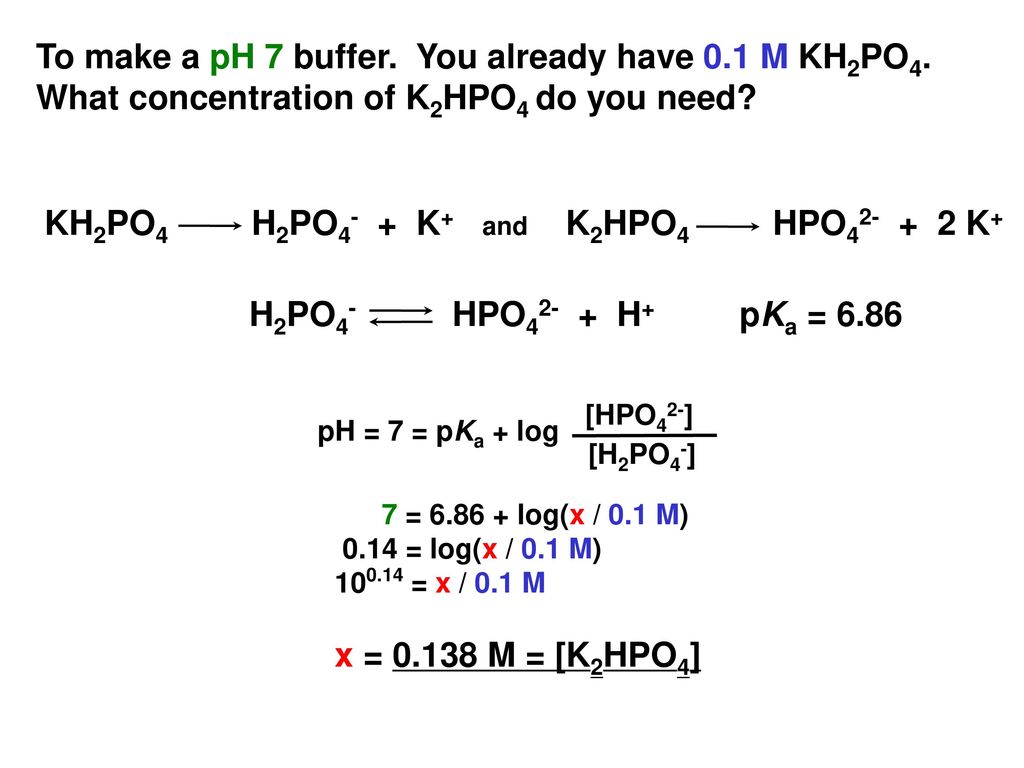
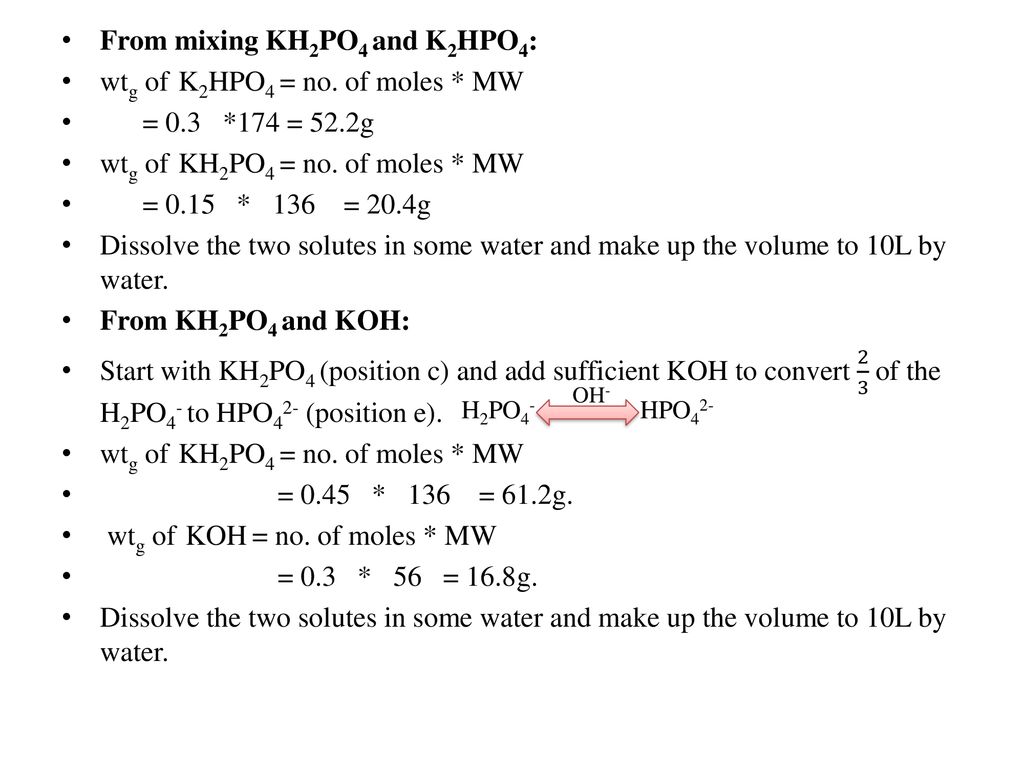
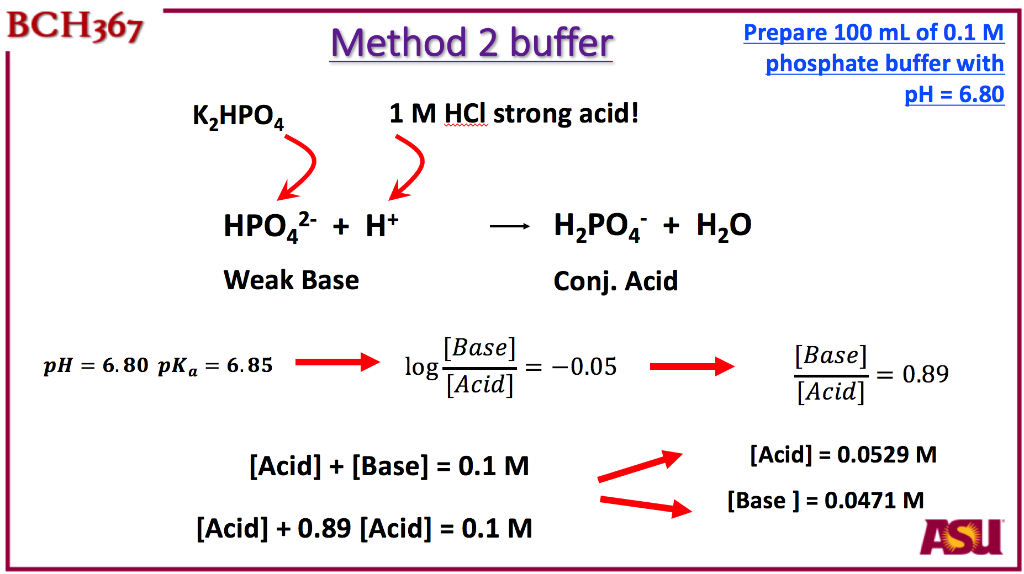
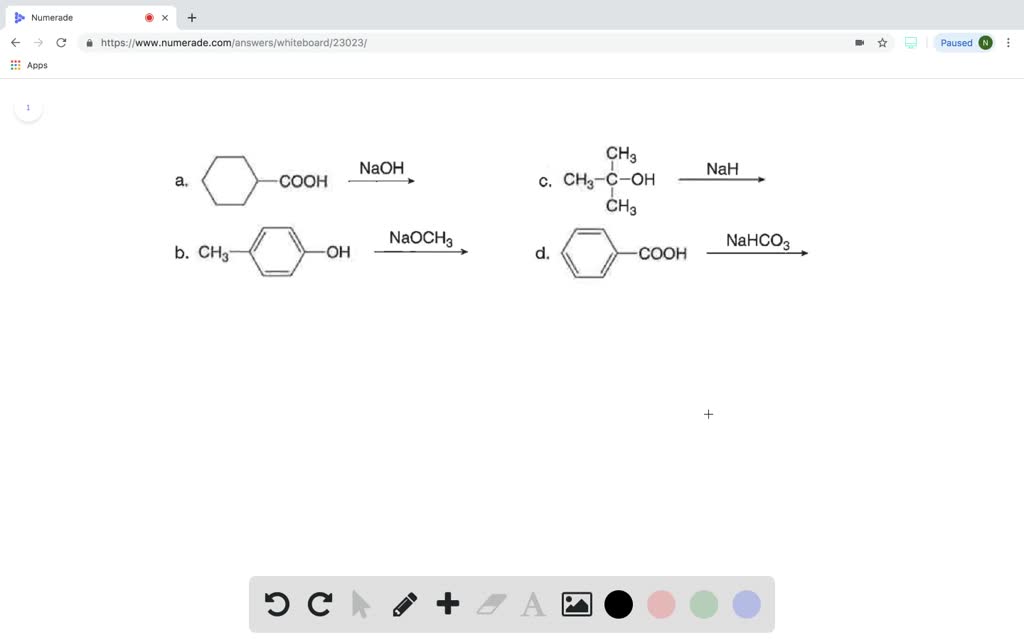
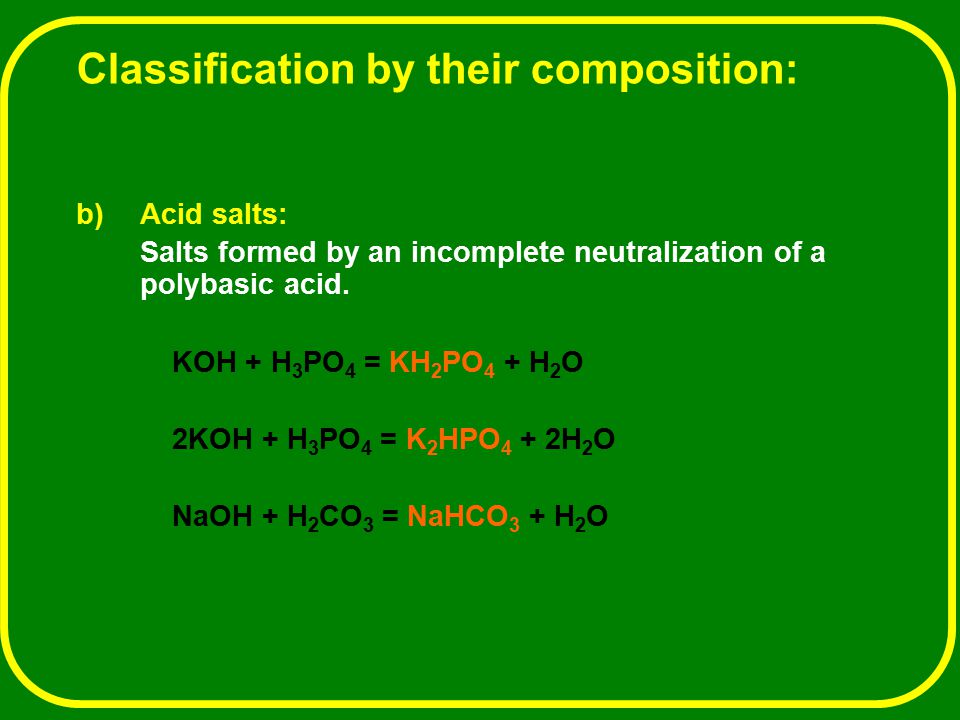
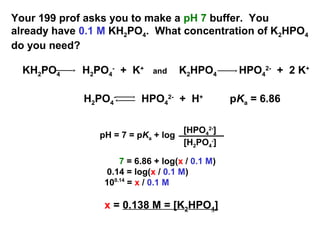
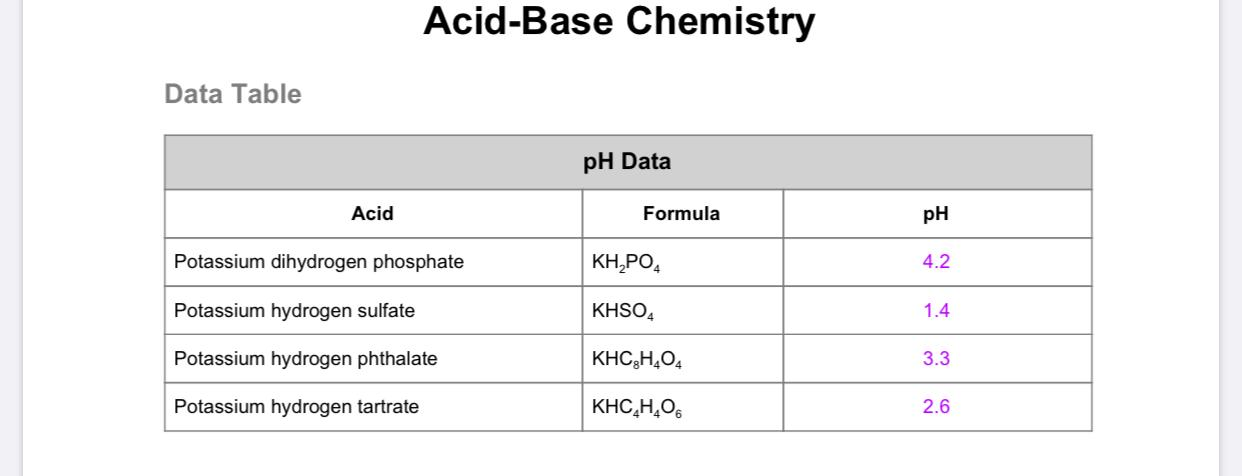
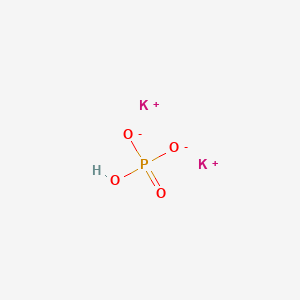
PRACTICE PROBLEMS FOR HE1 SUMMER 2005 1. Calculate the pH in each of the following solutions: (a) 1.86 x 10-3 M HBr (2.73)
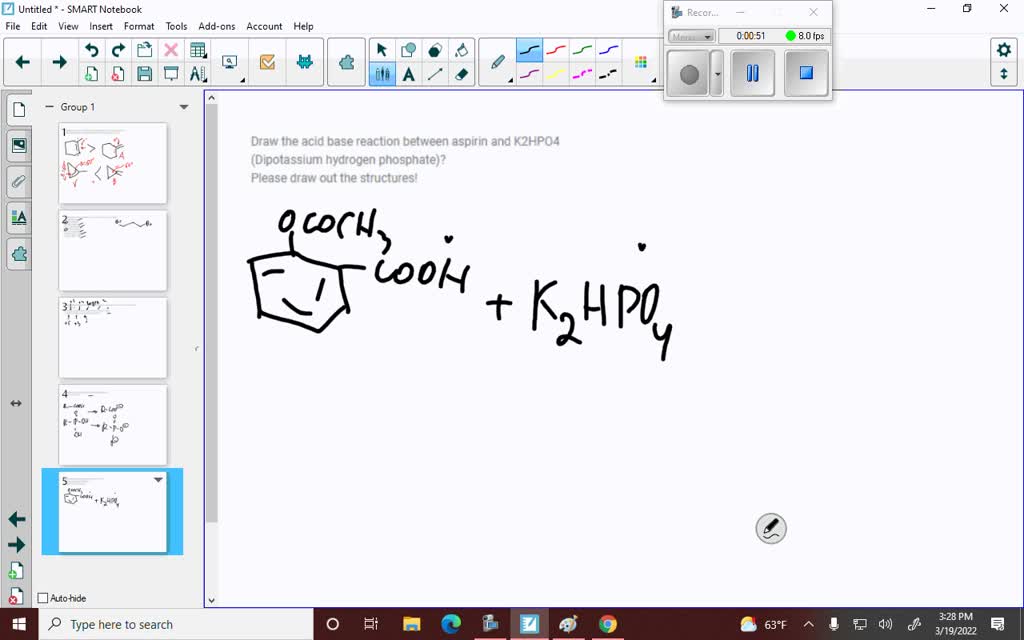
SOLVED: Draw the acid base reaction between aspirin and K2HPO4 (Dipotassium hydrogen phosphate)? Please draw out the structures!
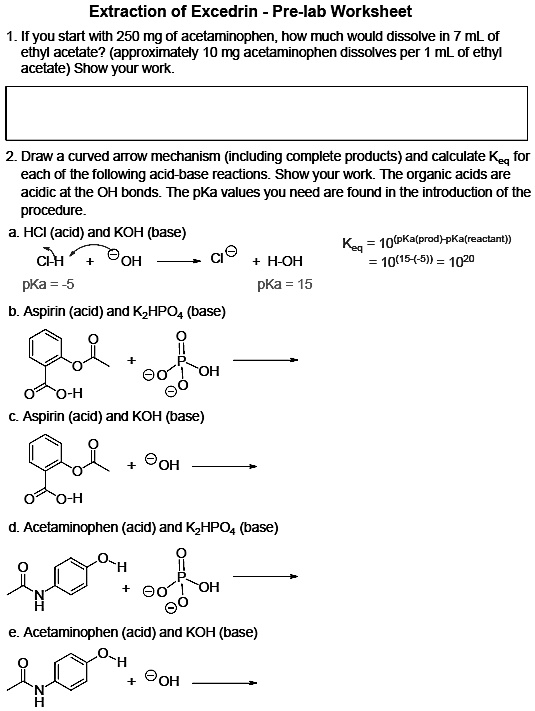
SOLVED: 'I am confused about the pKa and pH when it comes to reacting acid- base chemistry. :( Extraction of Excedrin Pre-lab Worksheet 1. If you start with 250 mg of acetaminophen, how