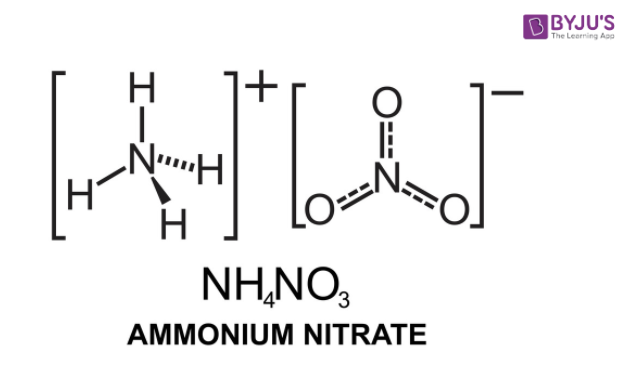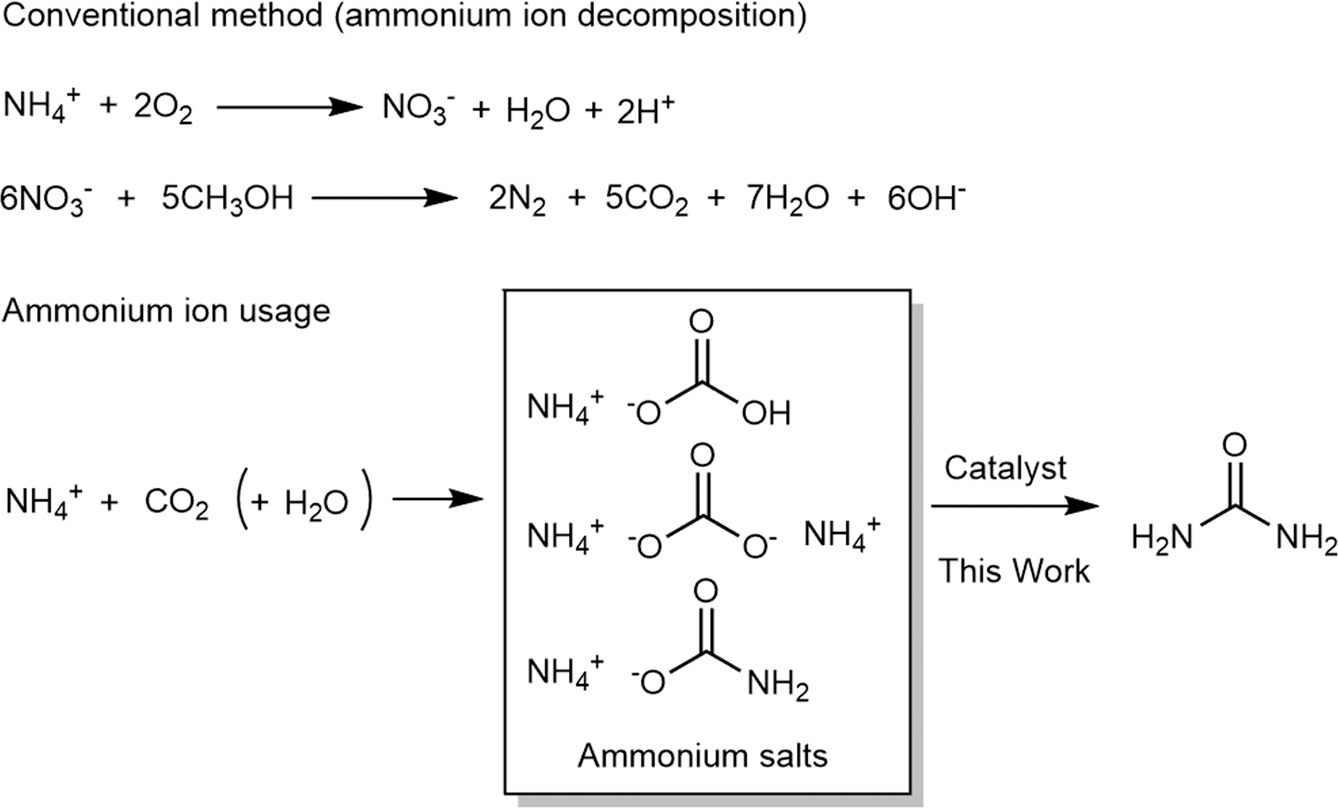
Organic bases catalyze the synthesis of urea from ammonium salts derived from recovered environmental ammonia | Scientific Reports

Write the predominant form for Butyl Ammonium is more water-soluble or more organically soluble. | Homework.Study.com
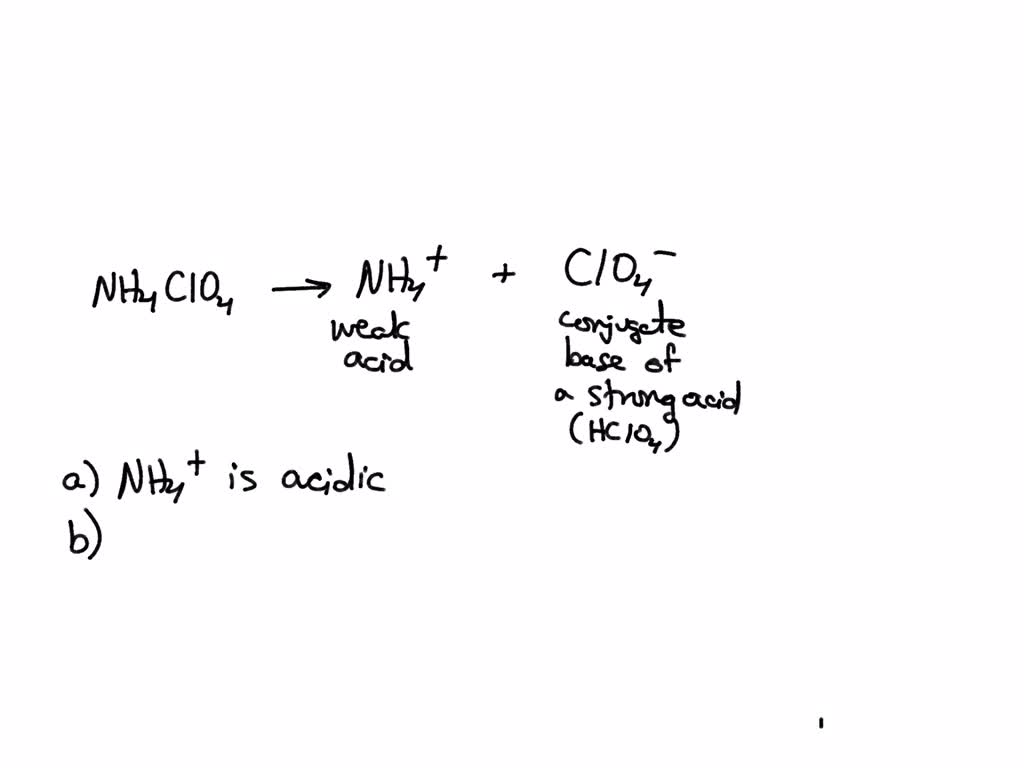
SOLVED: Consider the acid-base nature of ammonium perchlorate, NH4ClO4, when it is dissolved in water. (1) What are the acid-base properties of the cation? acidic/basic/neutral (2) What are the acid-base properties of
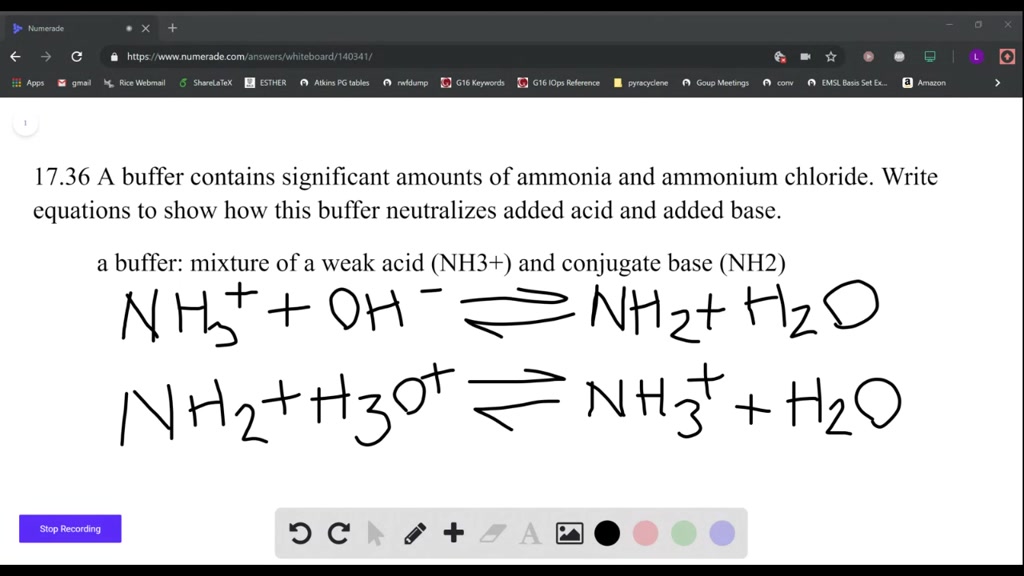
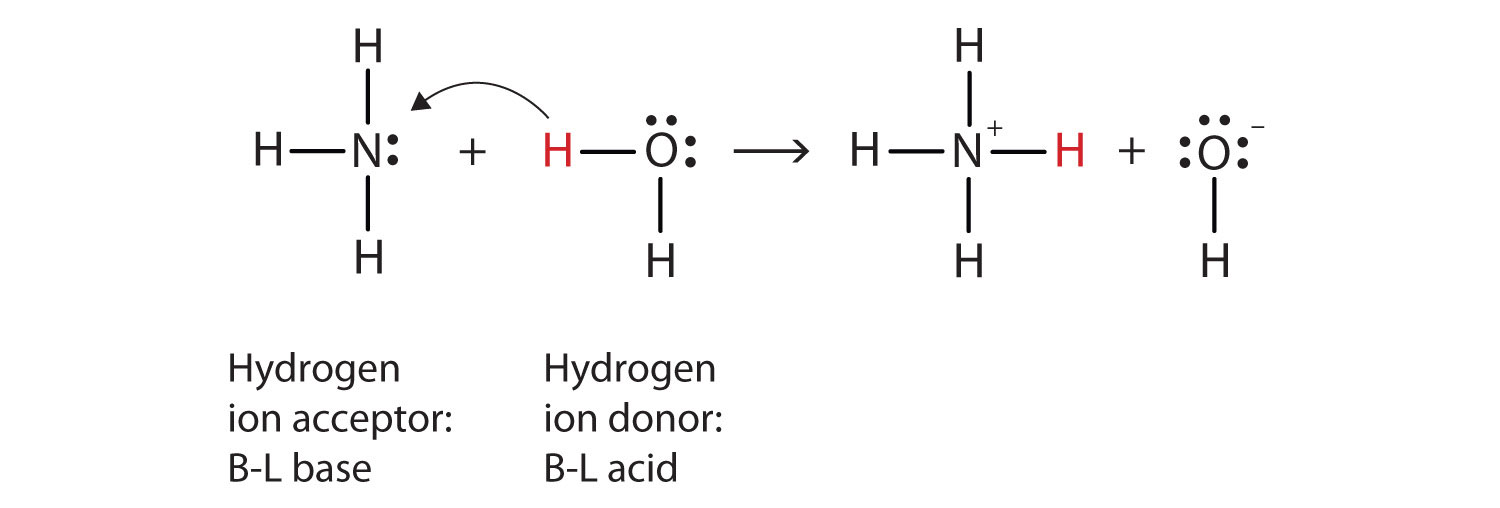
SOLVED: A buffer contains significant amounts of ammonia and ammonium ion. Write two equations, 1) showing how this buffer neutralizes added H3O+ and 2) showing how this buffer neutralizes added OH-.

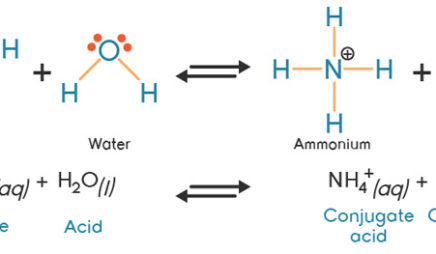
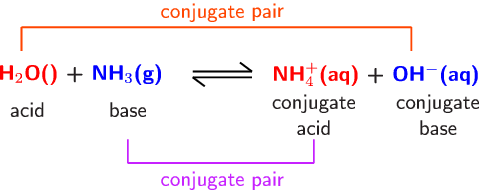
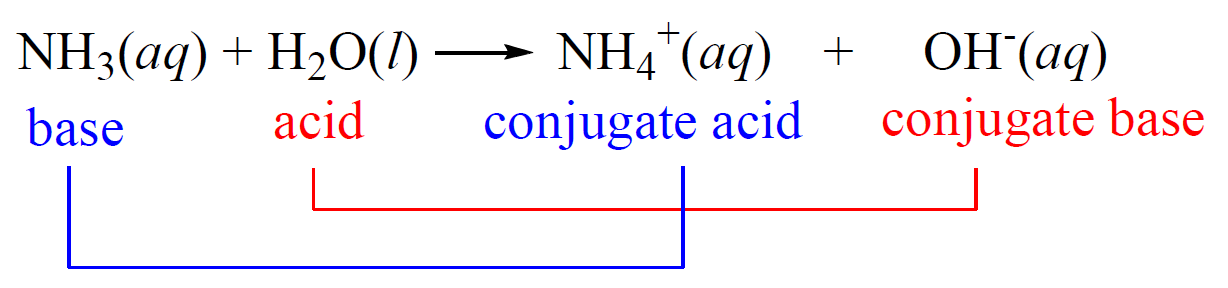
Ammonia is a weak base that reacts with water according to the equation NH(3)(aq)+H(2)O(l)hArrNH(4)^(+)(aq)+OH^(-)(aq) Select the correct option (s) that can increase the moles of ammonium ion in water: