Reaction of dC and 5MedC with hydroxylamine derivatives. Depiction of... | Download Scientific Diagram

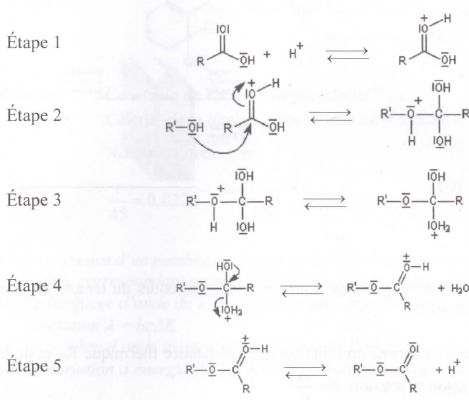
One‐Pot Synthesis of Hydroxamic Acids from Aldehydes and Hydroxylamine - Dettori - 2014 - Advanced Synthesis & Catalysis - Wiley Online Library
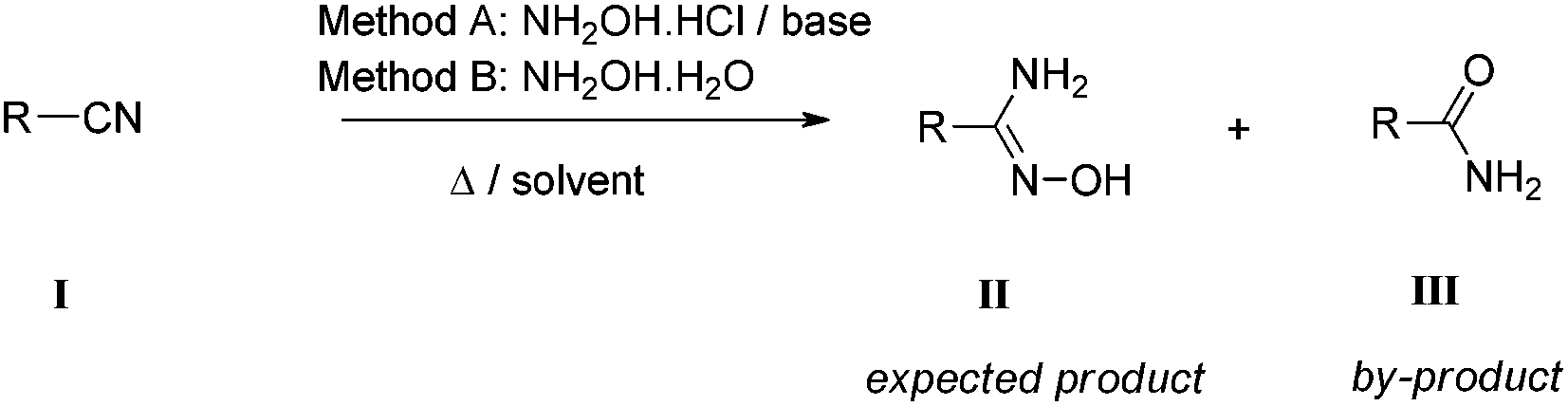
An experimental and theoretical study of reaction mechanisms between nitriles and hydroxylamine - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C4OB00854E
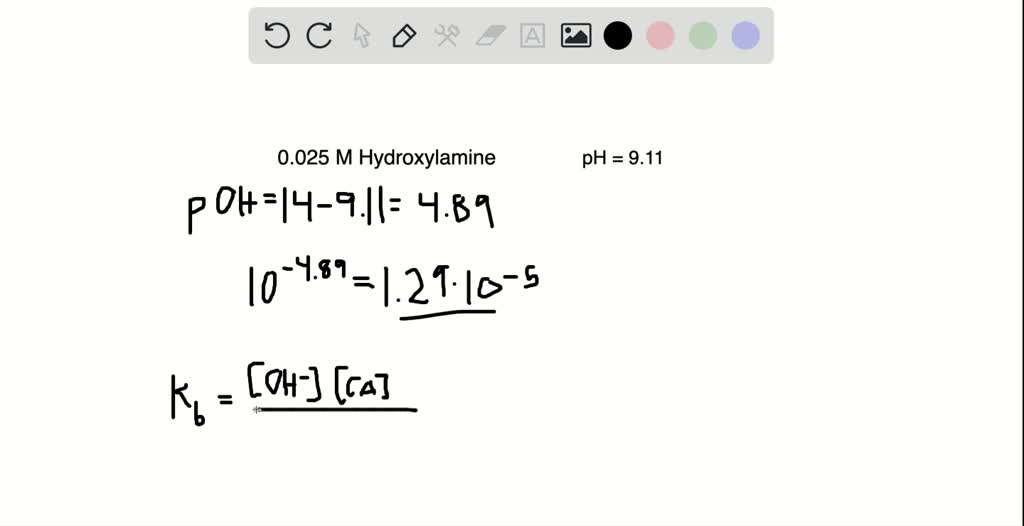
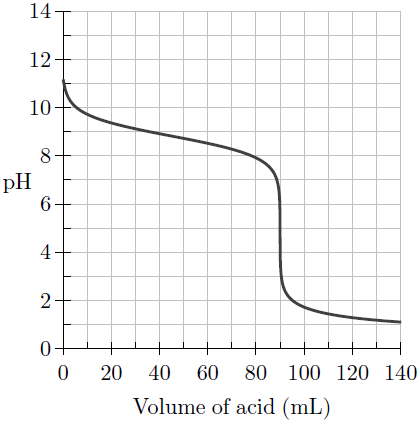
SOLVED:A 0.025 M solution of hydroxylamine has a pH of 9.11 What is the value of Kb for this weak base? H2 NOH(aq)+H2 O(ℓ) ⇄H3 NOH^+(aq)+OH^-(aq).
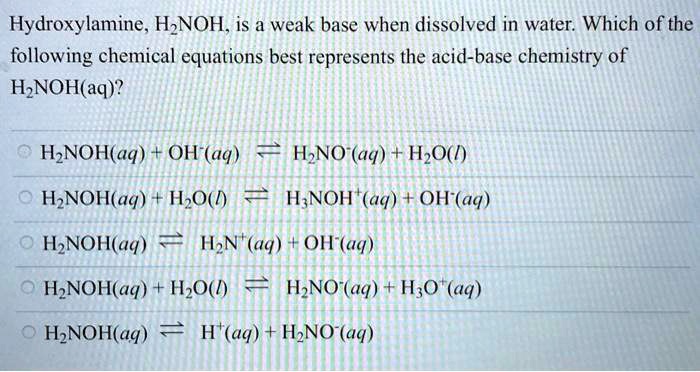
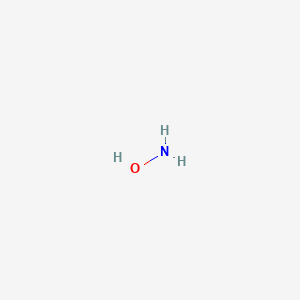
SOLVED: Hydroxylamine, HNOH, is a weak base when dissolved in water. Which of the following chemical equations best represents the acid-base chemistry of HNOH(aq)? a) HNOH(aq) + OH-(aq) b) HNO(aq) + H2O(l)

The Hydroxylamine Reaction of Sensory Rhodopsin II: Light-Induced Conformational Alterations with C13C14 Nonisomerizable Pigment: Biophysical Journal

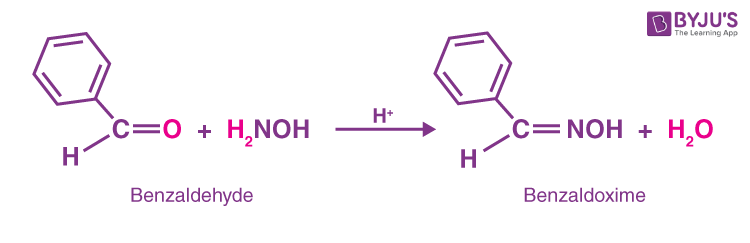
organic chemistry - Acetaldoxime Synthesis: Hydroxylamine vs Hydroxylamine HCl - Chemistry Stack Exchange

Formation of Aromatic Amidoximes with Hydroxylamine using Microreactor Technology | Organic Process Research & Development

Calculate the pH of a 0.050 M solution of hydroxylamine, NH2OH. (Kb = 6.6 x 10-9) | Homework.Study.com

An Integrated Process for the Synthesis of Solid Hydroxylamine Salt with Ammonia and Hydrogen Peroxide as Raw Materials | Industrial & Engineering Chemistry Research