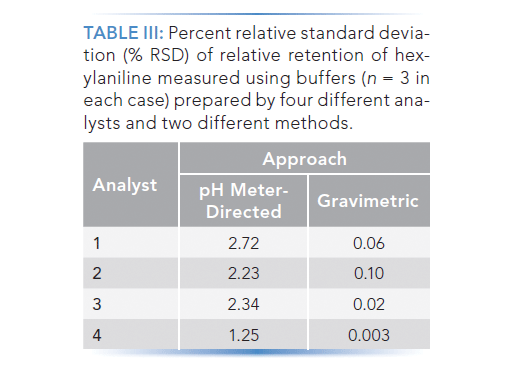
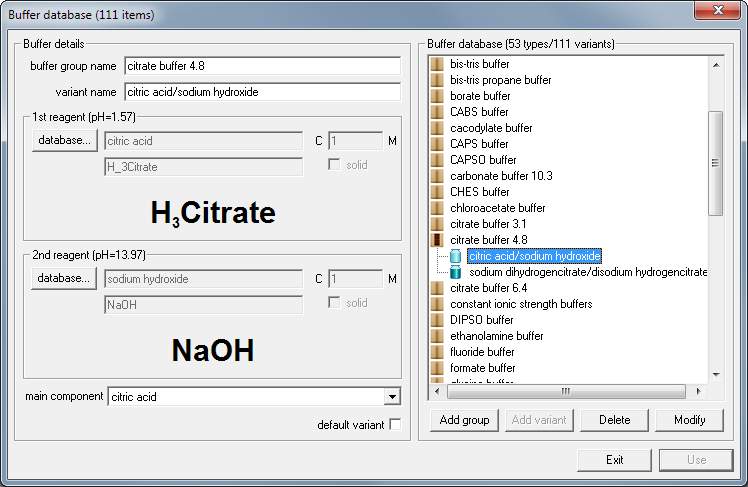
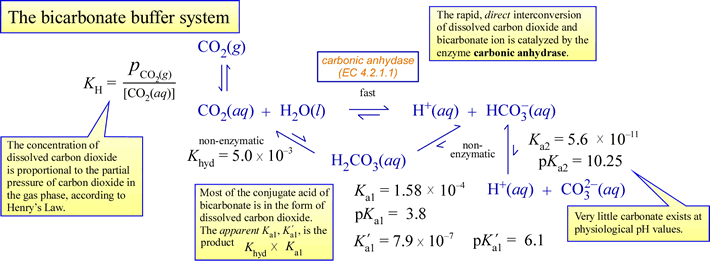
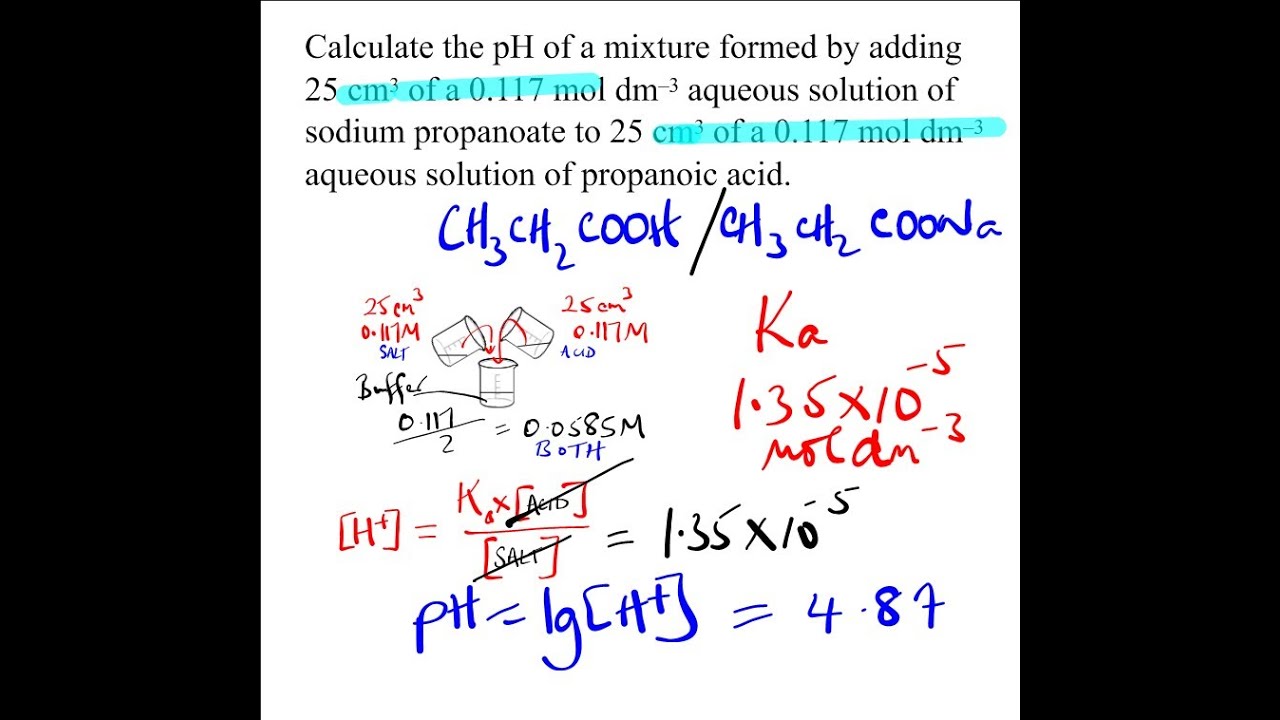
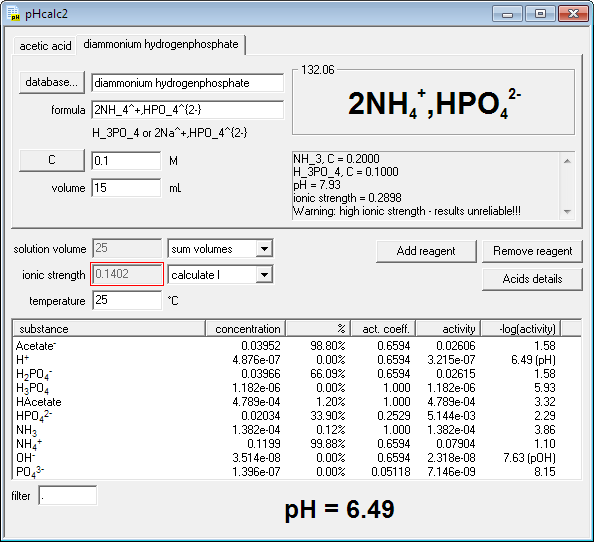
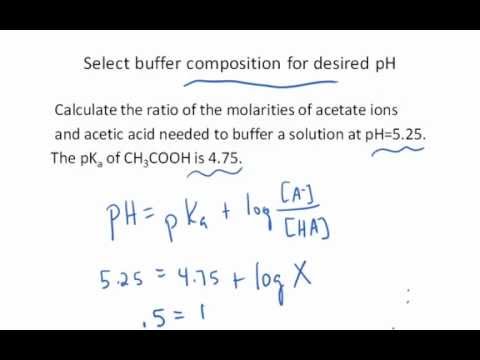
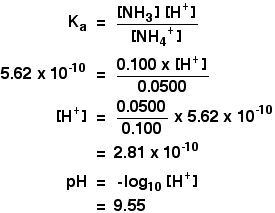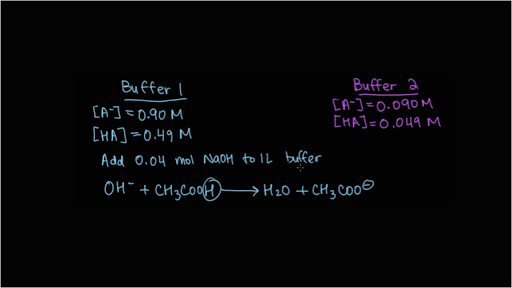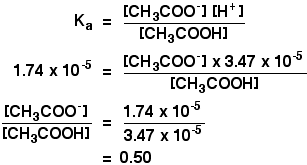SOLVED: You will be preparing a working solution of 1X using a stock solution of 100X buffer. Using the formula below, perform the calculation for preparing 250 mLs of a 1X working
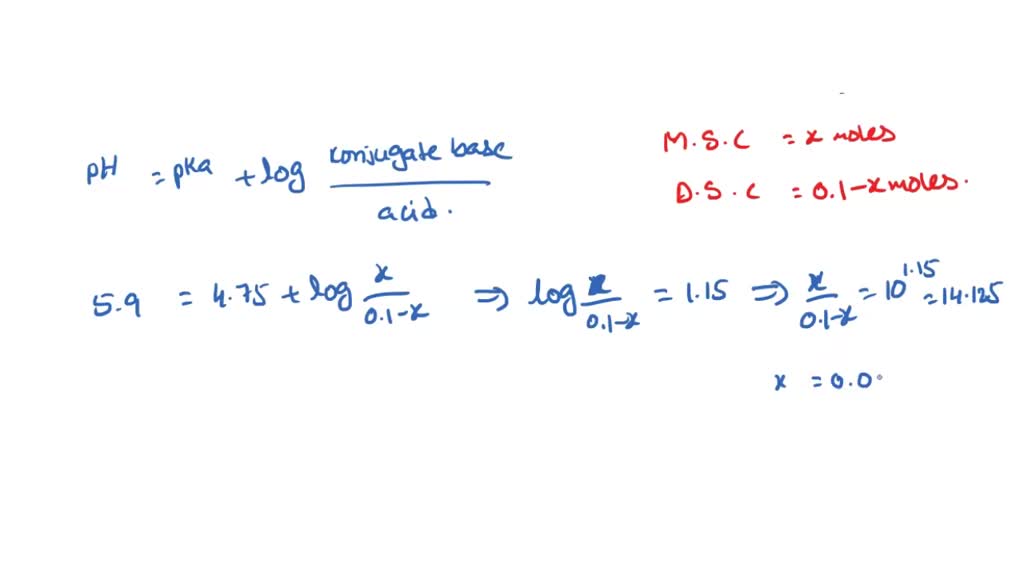
SOLVED: Describe the preparation of 1.0 L of 0.1 M citrate buffer, pH 5.90, starting with crystalline citric acid (FW = 210; pka1 = 3.1; pka2 = 4.75; pka3 = 5.4) and 1.0 M NaOH.