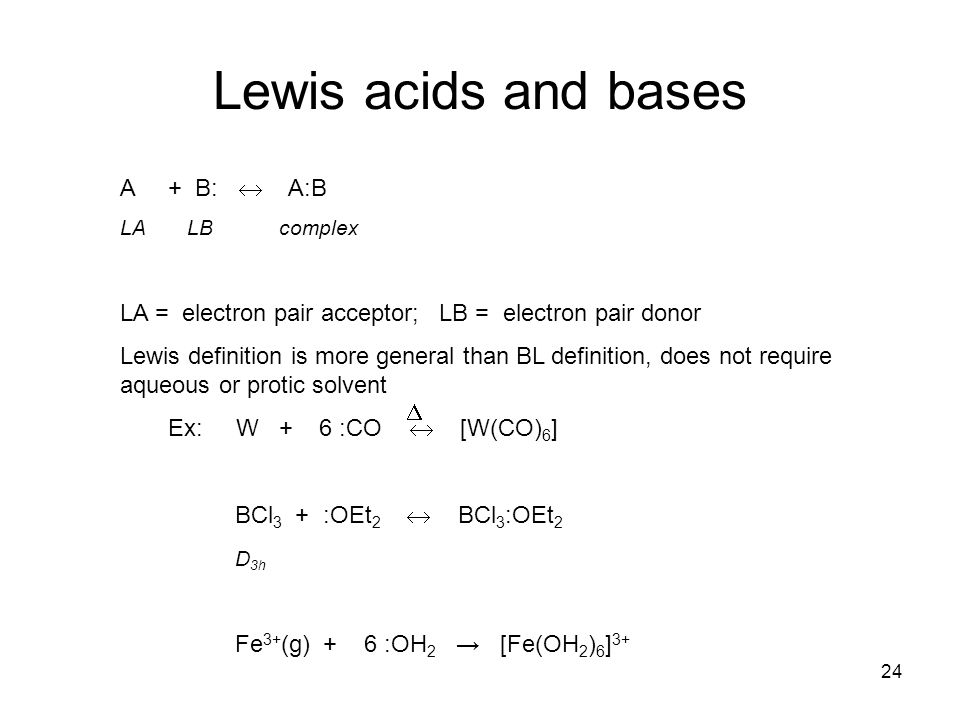
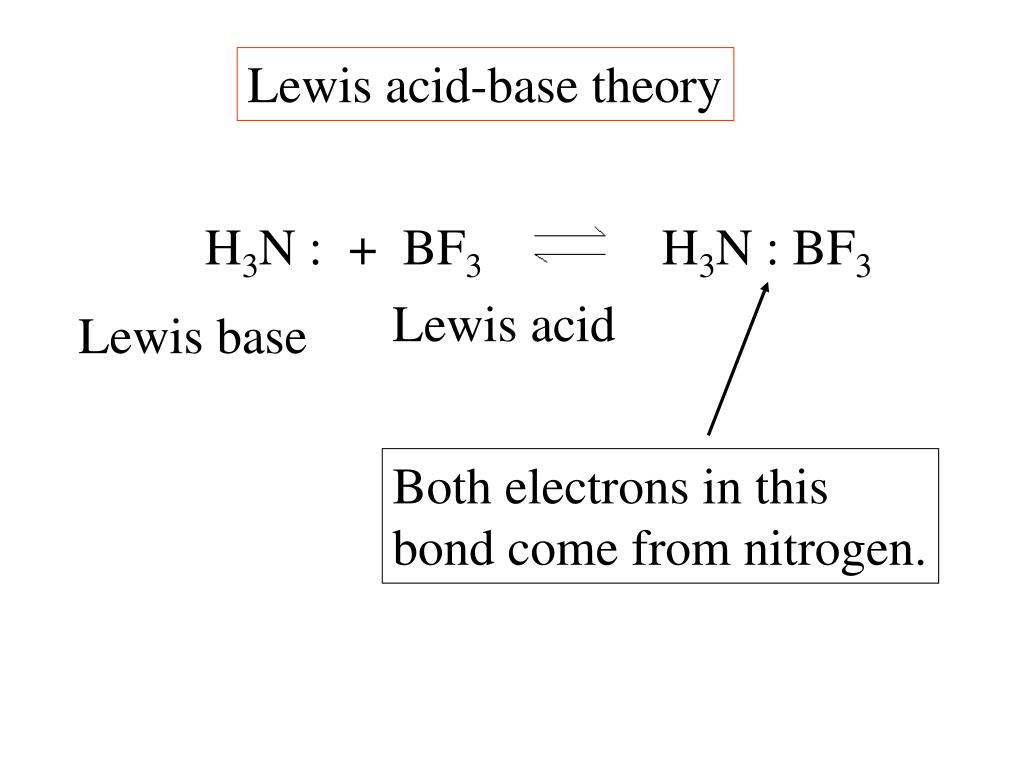
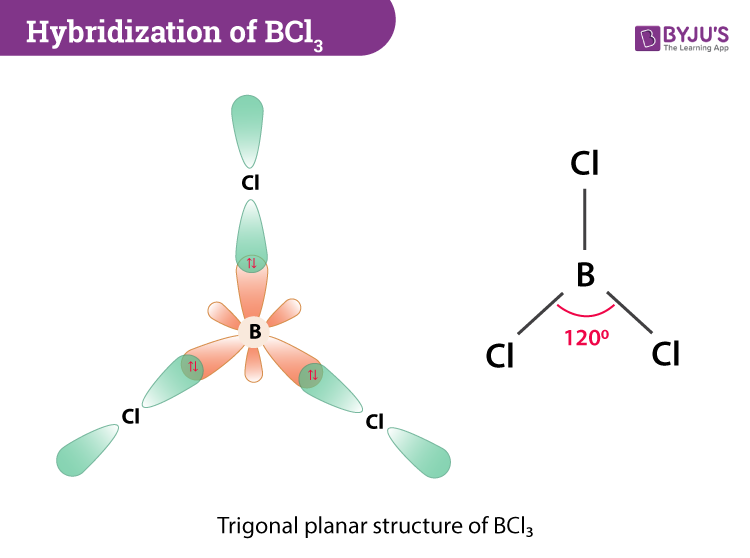
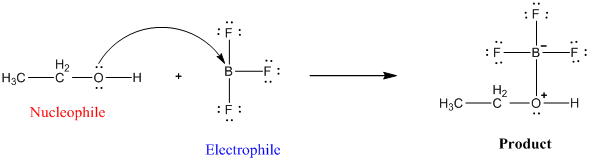
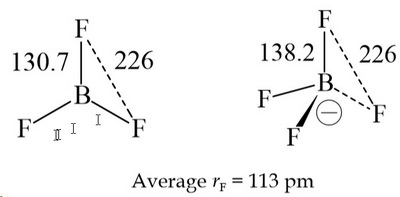
BCl3 lewis structure, molecular geometry, polar or nonpolar, hybridization, Bond angle in 2022 | Molecular geometry, Molecular, Vsepr theory

Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis ... - YouTube
![SOLVED: BCl3 + Cl- = [ BCl 4]- , in the equation which is the base? * a. BCl3 b. Cl c. BCl 4 d. none of these. SOLVED: BCl3 + Cl- = [ BCl 4]- , in the equation which is the base? * a. BCl3 b. Cl c. BCl 4 d. none of these.](https://cdn.numerade.com/ask_previews/39f5fa0a-dd33-4254-bfb2-4ac92f81a9c1_large.jpg)
SOLVED: BCl3 + Cl- = [ BCl 4]- , in the equation which is the base? * a. BCl3 b. Cl c. BCl 4 d. none of these.

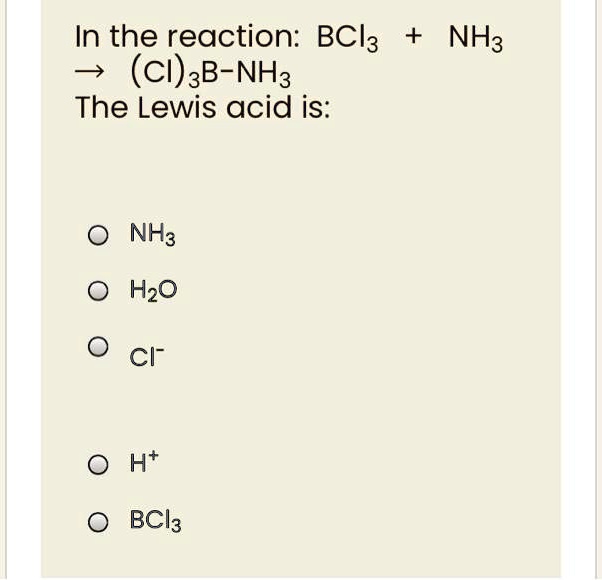
Decide whether the given substance should be classified as a Lewis acid or a Lewis base.BCl3 (Hint; Draw the Lewis dot structure.) | Homework.Study.com
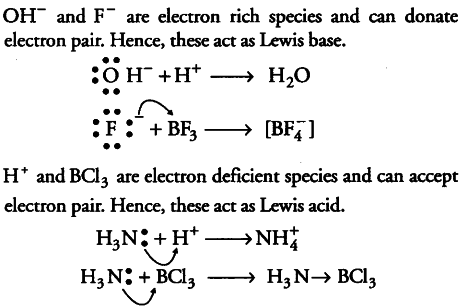
Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/base? - CBSE Class 11 Chemistry - Learn CBSE Forum

SOLVED: Identify the Lewis acids and Lewis bases: BCl3 +Ci = BCIA" The Lewis base is CI^ The Lewis acid is BCI3 SO2 + H20 = H2803 The Lewis base is H2O