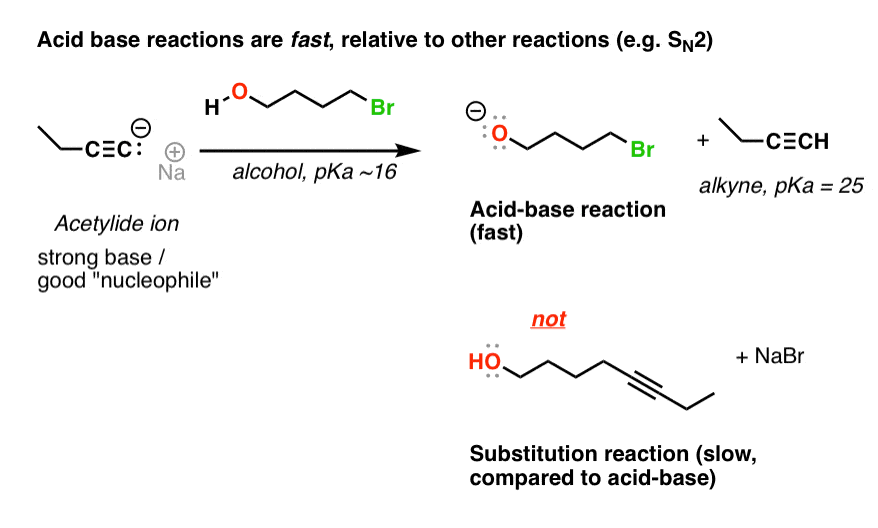
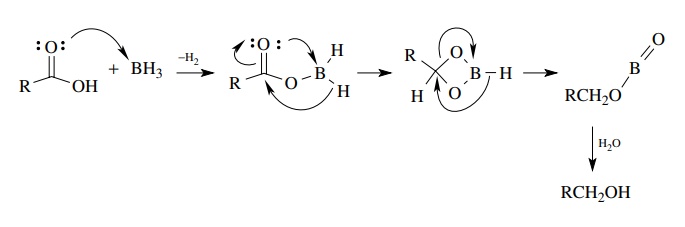
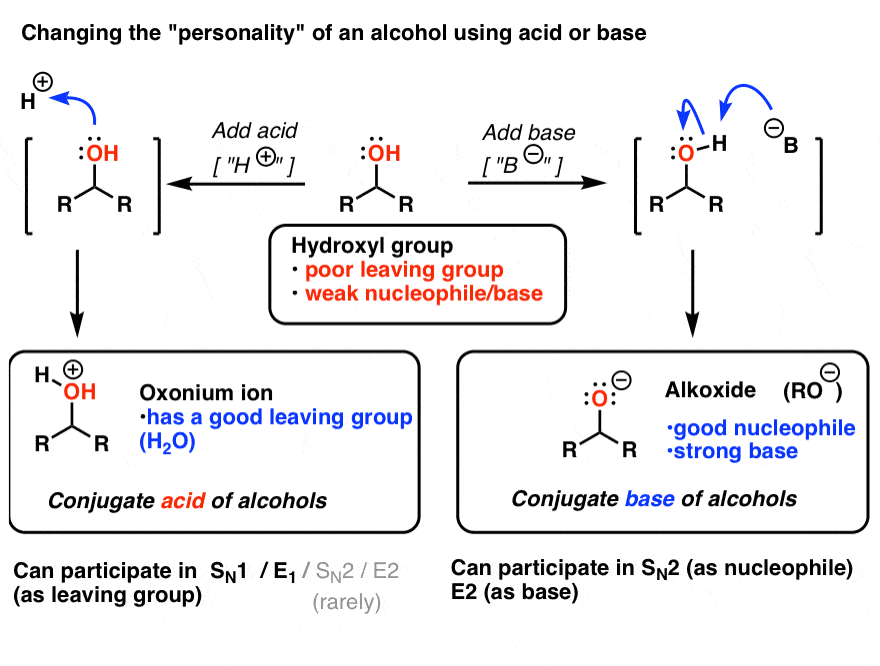
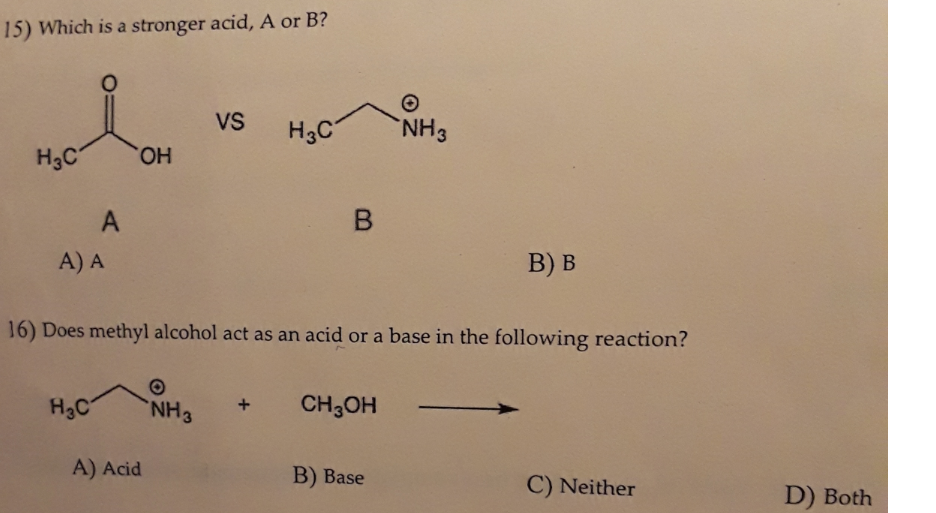
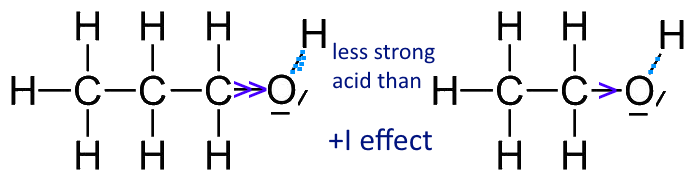
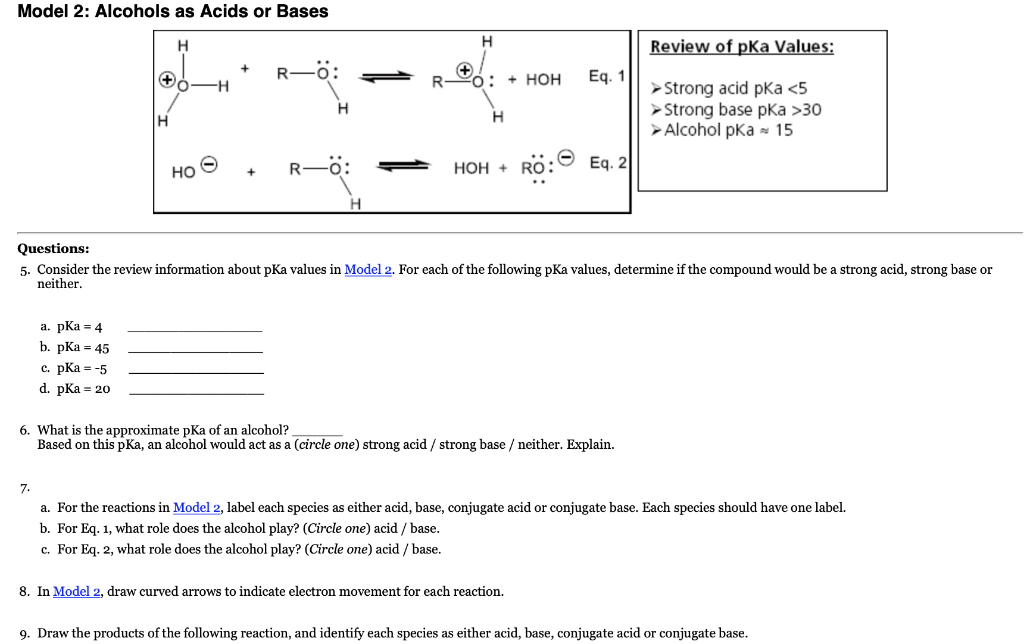
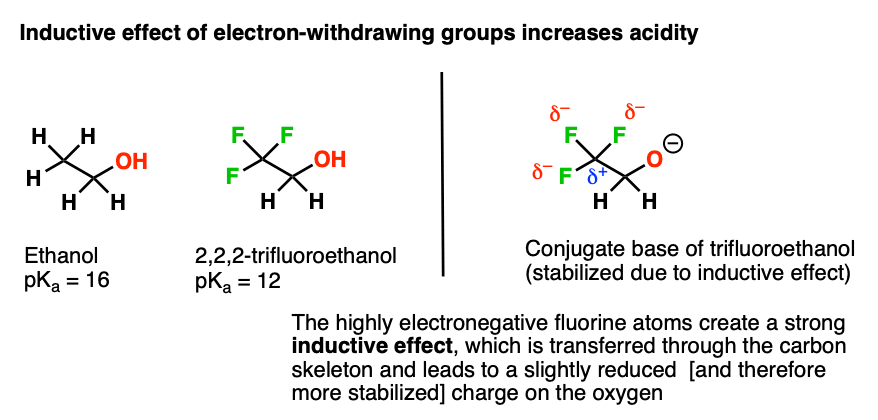
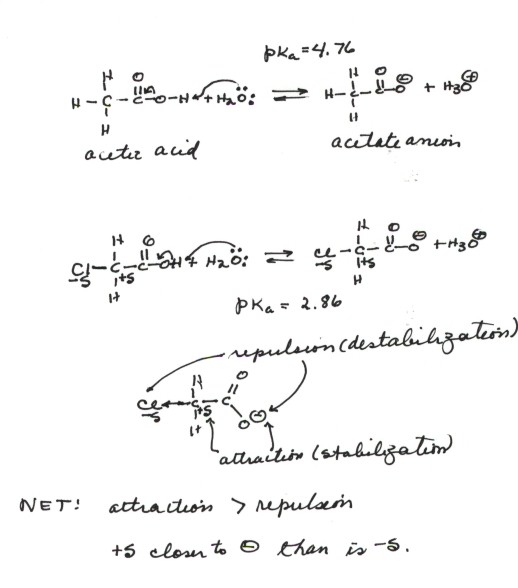
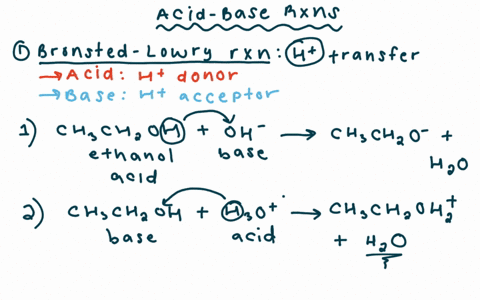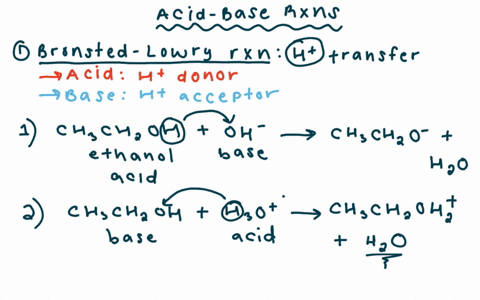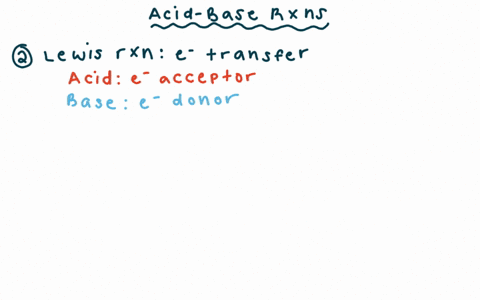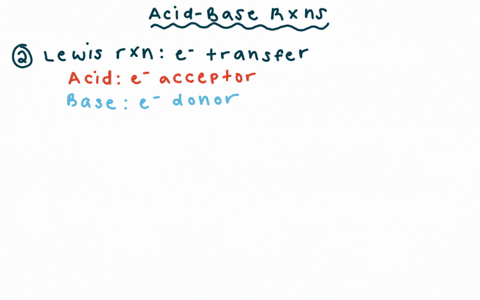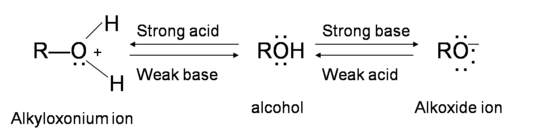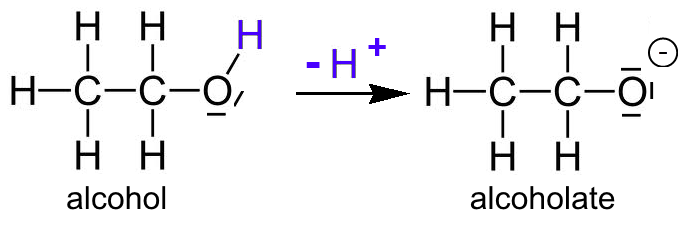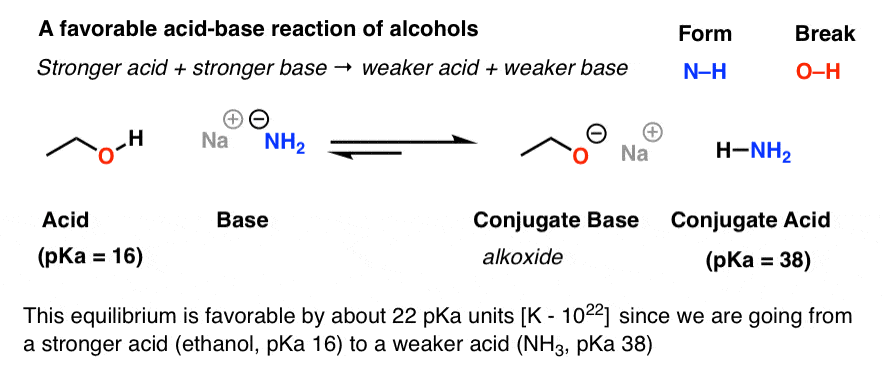Why is the conjugate acid of an ether/alcohol more acidic than that of a hydronium ion? : r/chemhelp

The hydride ion H^- is stronger base than hydroxide ion OH^- . Which of the following reaction will occur if sodium hydride (NaH) is dissolved in water?

organic chemistry - Why can alcohol undergo elimination just by sulphuric acid? - Chemistry Stack Exchange