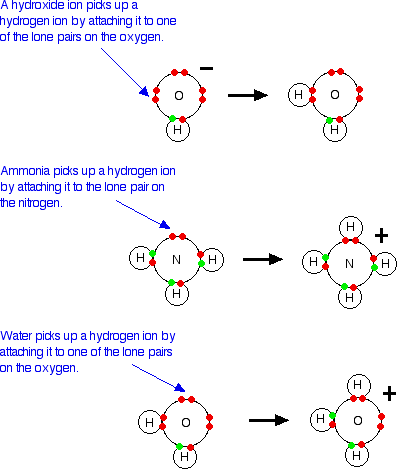
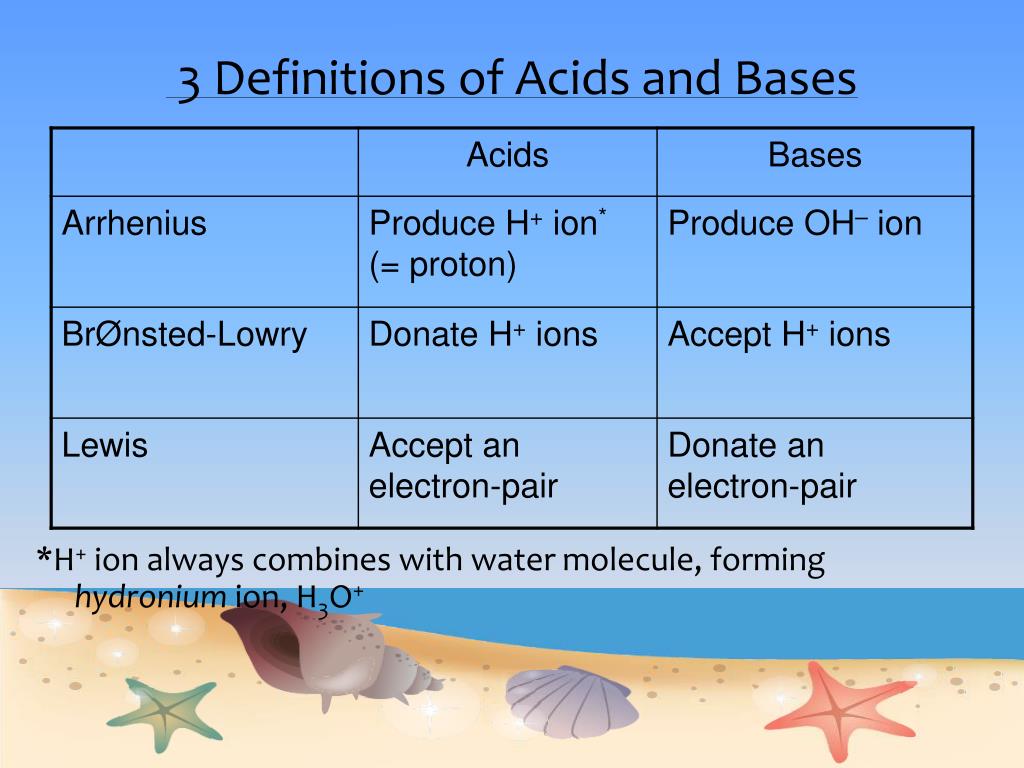
Definitions. Arrhenius Acids and Bases Acids release hydrogen ions in water. Bases release hydroxide ions in water. An acid is a substance that produces. - ppt download
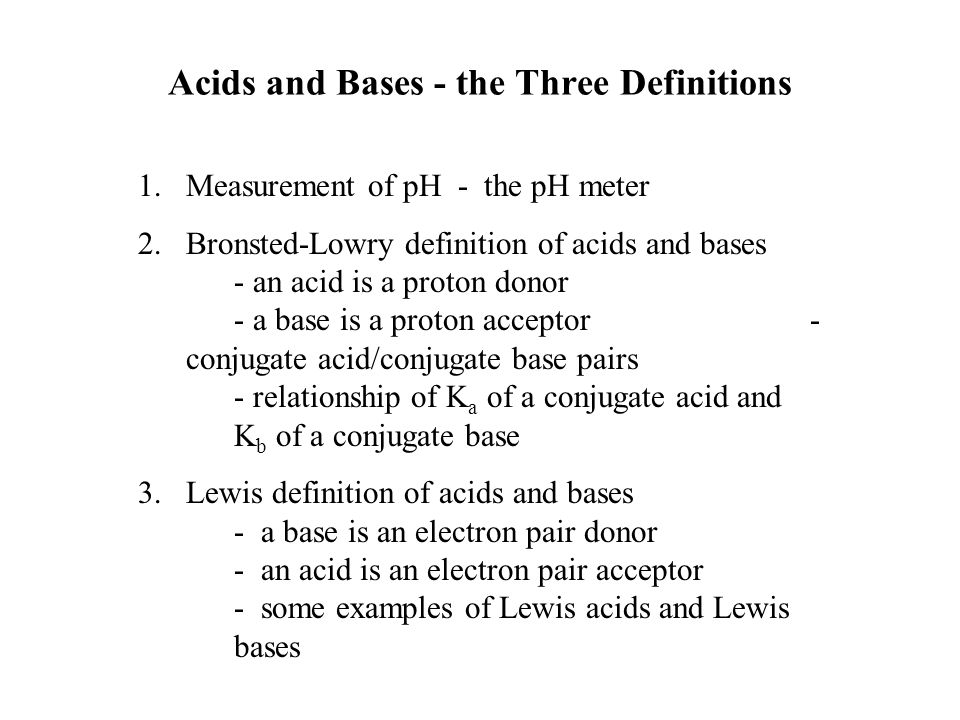
Acids and Bases - the Three Definitions 1.Measurement of pH - the pH meter 2.Bronsted-Lowry definition of acids and bases - an acid is a proton donor - - ppt download

Acids and Bases. Different Definitions of Acids and Bases Arrhenius definitions for aqueous solutions. acid: acid: a substance that produces H + (H ppt download

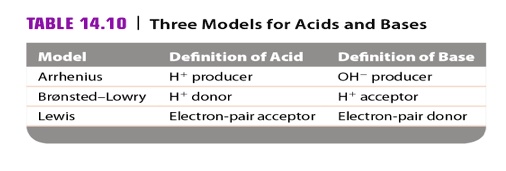
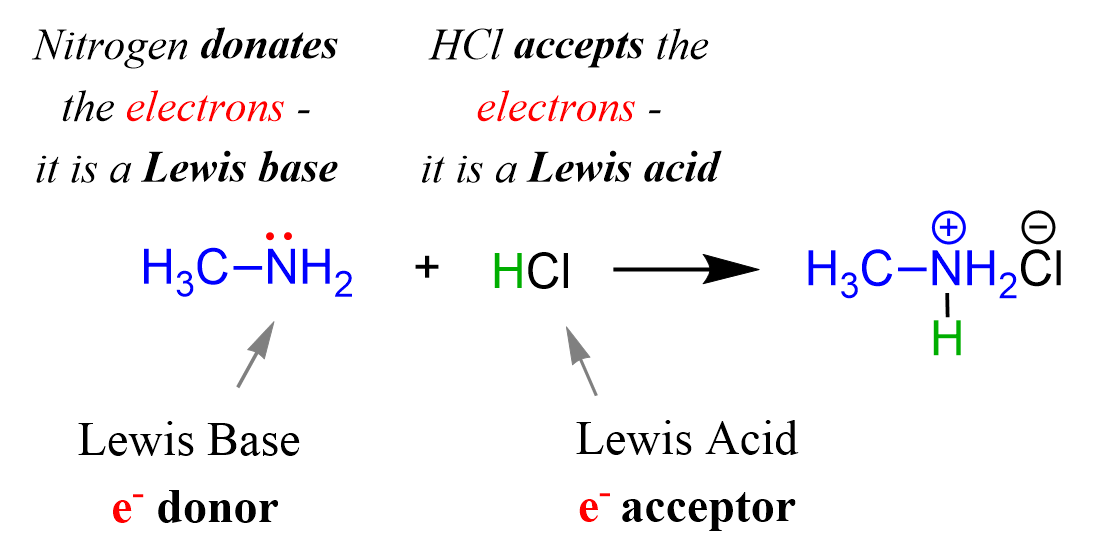
Acids and Bases 3 definitions for acids and bases – Arrhenius – Bronsted-Lowry – Lewis Must be in solution – Most often dissolved in water (aqueous) Inorganic. - ppt download

1 Capsaicin. 2 Chapter 2 ACIDS and BASES 3 Definitions of Acid-Base Arrhenius : acid dissociates in aqueous solution to form H 3 O + base dissociates. - ppt download








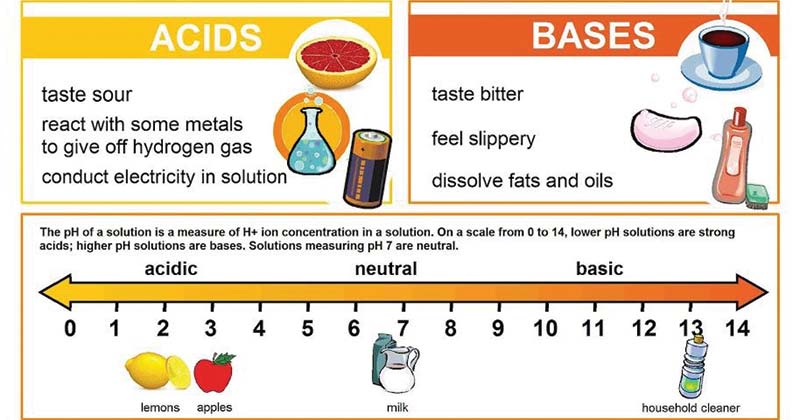

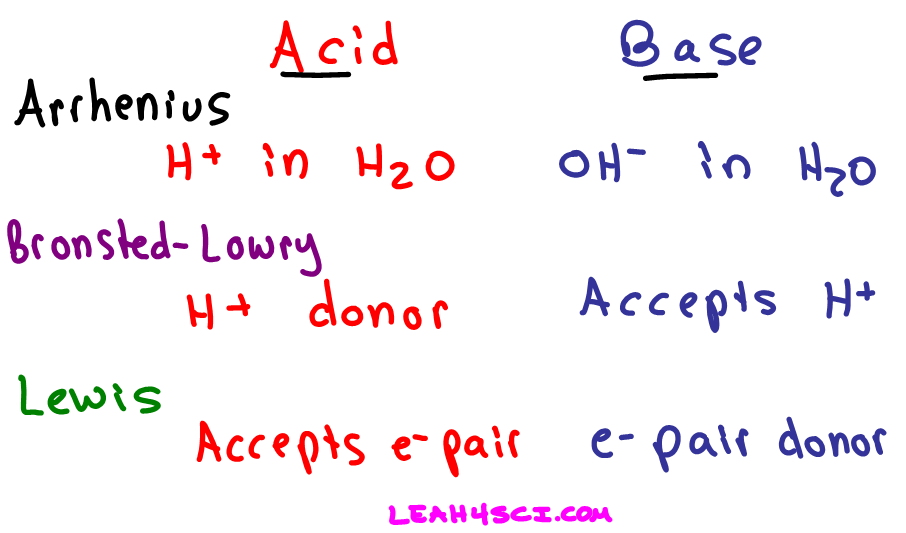
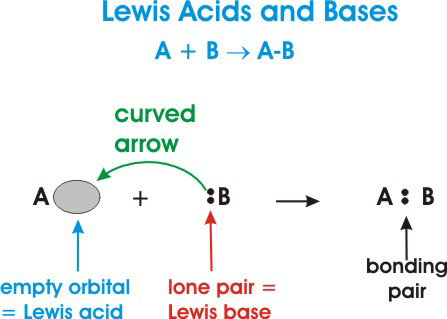
:max_bytes(150000):strip_icc()/one-ambidextrous-indian-man-writing-chemical-equation-on-greenboard-154948585-5880bed45f9b58bdb32f7efb.jpg)